Abstract
Nucleoporins are a specialized subset of nuclear proteins that comprise the nuclear pore complex and regulate nucleocytoplasmic transport. Recent demonstrations of roles for individual nucleoporins in multiple paradigms of differentiation via mechanisms independent of nuclear trafficking represent conceptual advances in understanding the contributions of nucleoporins to cellular development. Among these, a functional role for nucleoporins in reproductive fitness and gametogenesis has been identified, supported by robust models and clinical studies that leverage the power of next generation sequencing technology to identify reproductive-disease-associated mutations in specific nucleoporins. Proper nucleoporin function manifests in different ways during oogenesis and spermatogenesis. However, nonhuman models of gametogenesis may not recapitulate human mechanisms, which may confound translational interpretation and relevance. To circumvent these limitations, identification of reproductive pathologies in patients, combined with next generation sequencing approaches and advanced in silico tools, offers a powerful approach to investigate the potential function of nucleoporins in human reproduction. Ultimately, elucidating the role of nucleoporins in reproductive biology will provide opportunities for predictive, diagnostic, and therapeutic strategies to address reproductive disorders.
Keywords: nucleoporins, oogenesis, spermatogenesis, nuclear pore complex, reproduction
Résumé :
Les nucléoporines constituent un sous-ensemble spécialisé de protéines nucléaires, qui comprennent le complexe des pores nucléaires et participent à la régulation du transport nucléo-cytoplasmique. De récentes démonstrations des rôles de nucléoporines données dans de multiples paradigmes de différentiation par l’intermédiaire de modes d’action indépendants des échanges nucléaires correspondent à des avancées conceptuelles dans la compréhension des contributions de nucléoporines au développement de la cellule. Parmi celles-là, on a établi que les nucléoporines jouent un rôle fonctionnel dans la santé de la reproduction et la gamétogenèse, observations appuyées par des modèles et des études cliniques robustes qui tirent profit de la puissance de nouvelles générations de technologies de séquençage en vue de déceler des mutations de nucléoporines spécifiques associées à des maladies de la reproduction. Le bon fonctionnement des nucléoporines se manifeste de différentes manières au cours de l’ovogenèse et de la spermatogenèse. Cependant, les modèles de gamétogenèse non humains pourraient ne pas reprendre les modes d’action chez l’humain, ce qui pourrait contrecarrer l’interprétation et la pertinence translationnelles. En vue de contourner ces limites, relever des pathologies reproductives chez les patients, en association avec de nouvelles générations d’approches de séquençage et des outils informatiques évolués, offre une approche puissante pour l’étude d’éventuelles fonctions des nucléoporines dans la reproduction humaine. Ultimement, élucider le rôle des nucléoporines dans la biologie de la reproduction fournira l’occasion d’établir des stratégies prédictives, diagnostiques et thérapeutiques en vue de s’attaquer aux troubles de la reproduction.
Mots-clés: nucléoporines, ovogenèse, spermatogenèse, complexe des pores nucléaires, reproduction
Introduction
Mounting evidence supports the paradigm of specific nucleoporins as noncanonical epigenomic regulators of differentiation and disease (Capelson and Hetzer 2009; Ibarra et al. 2016). Alternative to their role as facilitators of nucleocytoplasmic traffic, nucleoporins exert direct and indirect effects at genomic and transcriptomic levels to secure diverse cell fates (Gomez-Cavazos and Hetzer 2015; Jacinto et al. 2015; Raices and D’Angelo 2012). Genetic and biochemical studies have identified germline and gametogenic robustness dependent on nucleoporin stability (Akiyama et al. 2013; Park et al. 2016), underscored by gonadal expression and cellular localization dynamics during reproduc tive development (Popken et al. 2016). These observations reinforce the hypothesis that nucleoporin (dys)functions underlie metazoan reproductive biology (Weinberg-Shukron et al. 2015), and the precise mechanisms of how nucleoporins accomplish this is a rich area of investigation.
Nucleoporins
Nucleoporins are a class of specialized proteins that form the nuclear pore complex (NPC), a macromolecular structure that is highly conserved from yeast to higher eukaryotes (Beck and Hurt 2017). The NPC mediates a GTP-facilitated molecular transport mechanism that shuttles cargoes >40 kDa in size between the nuclear and cytosolic compartments (Gomez-Cavazos and Hetzer 2015). These cargoes include different classes of RNA molecules and proteins that utilize distinct receptor-mediated mechanisms of transport. The variety of nuclear transport receptors, i.e., karyopherins, that control trafficking participates in an intricate process that integrates soluble transport machinery, e.g., karyopherins, RanGTPase, RanGEF, with the nuclear envelope-localized NPC to regulate gene expression (Ritterhoff et al. 2016; Yaseen and Blobel 1999).
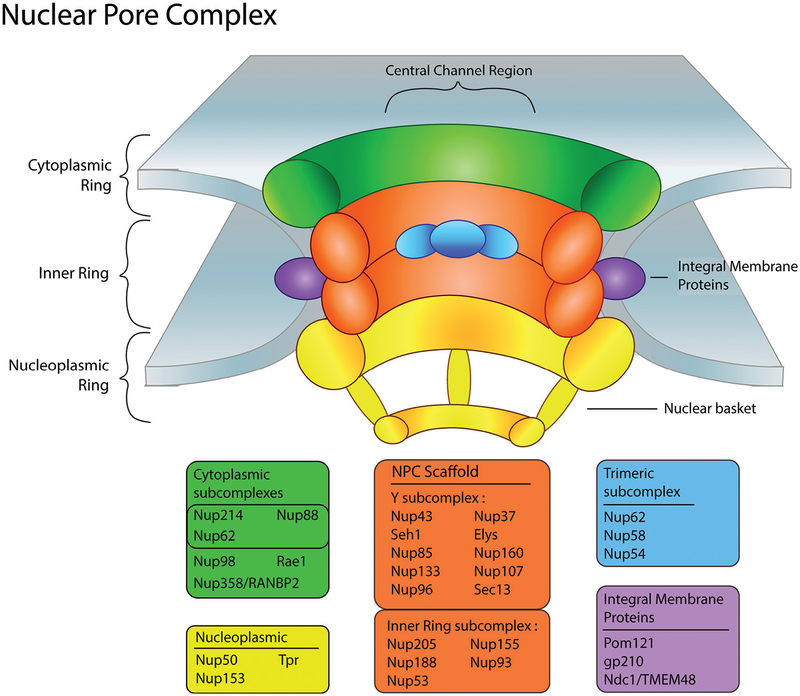
The NPC has been shown to be evolutionarily conserved and although the general mechanism of how molecules move through the pore is well understood, precise details of molecular transit are still uncharacterized (von Appen and Beck 2016). Structurally, the NPC is composed of approximately 30 discrete nucleoporins constitutively organized to create well-defined cytoplasmic, inner, and nuclear regions within the NPC (Fig. 1) (Beck and Hurt 2017; von Appen and Beck 2016). Within these regions, multiple copies of nucleoporins are stacked and linked together to form subcomplexes to establish NPC domains critical for nuclear transport. Molecular cargo then interacts with sites at the cytoplasmic face or with the nuclear basket structure of the NPC prior to transiting through the central channel (Fig. 1).
Fig. 1.
Nuclear pore complex (NPC) structural components. Illustration of the different regions that comprise the NPC: cytoplasmic, nucleoplasmic, and inner rings. Multiple copies of nucleoporins assemble within these regions in a stack-like manner forming subcomplexes. The nucleoporins are separated into corresponding color-coded boxes that signify each subcomplex and their localization within the NPC. Elys, embryonic large molecule derived from yolk sac; gp210, nuclear pore membrane glycoprotein 210; Nup, nucleoporin; Rae1, ribonucleic acid export 1; RANBP2, Ran binding protein 2; Pom121, nuclear envelope pore membrane protein of 121 kDa; TMEM48, transmembrane protein 48; Tpr, translocated promoter region.
Role of nucleoporins in development
Individual nucleoporins have demonstrated gene regulation of pluripotency independent of their canonical role in architectural support and facilitated transport functions of the NPC (Gomez-Cavazos and Hetzer 2015). For example, analysis of an embryonic stem (ES) cell line deficient in the NPC inner ring subcomplex nucleoporin, Nup155, revealed global dysregulation of gene expression in Nup155 compromised ES cells (Preston et al. 2018). Abnormal gene over- and under-expression in the Nup155-insufficient ES cell line led to reprioritized functional gene ontologies that included membrane biology and RNA metabolism. Furthermore, the splicing factor Srsf2 was identified as essential to the overall architecture of a cardiogenic gene subnetwork (Preston et al. 2018). Previous work in which conditional knockout of Srsf2 in ventricular tissue leads to poor calcium handling upon increased pacing has been reported (Ding et al. 2004), in line with the effects observed on contractile embryoid bodies derived from Nup155-deficient ES cells (Preston et al. 2018). This is supported by earlier observations of a clinical mutation for NUP155 associated with incidences of sudden cardiac death in a consanguineous pediatric cohort (Zhang et al. 2008).
Independent work examining direct regulatory interactions of nucleoporins with chromatin support the observed effects of nucleoporin deficiency on transcriptome remodeling. In a study that looked at the nuclear basket nucleoporin Nup153 and its potential to regulate gene expression by interacting directly with chromatin, discrete sites of Nup153 enrichment were found throughout the genome at specific intergenic super enhancer regions (Ibarra et al. 2016). Furthermore, these interactions with these super enhancer regions occurred at the nuclear periphery, supported by previous evidence indicating gene regulatory functions of NPC at the nuclear envelope (Popken et al. 2016). These NPC proximal and distal regulatory modalities suggest that discrete nucleoporins can facilitate gene regulation, and that regulation in the context of cell fate specification is more dynamic and intricate than first anticipated.
In addition to direct interactions with chromatin, nucleoporins may exert regulatory effects on developmental programming through indirect mechanisms. In a study conducted on mouse ES cells, it was observed that pluripotency was maintained by polycomb-repressive complex 1 (Prc1) recruitment to multiple and specific transcriptional start sites via Nup153 (Jacinto et al. 2015). Furthermore, Nup153 was downregulated during constitutive neural differentiation, whereas Nup153 knockout mice maintained high levels of multiple ectodermal markers that prevented neurogenesis, precluding cellular ability to acquire terminal identity (Jacinto et al. 2015).
A dramatic example of nonstandard roles for nucleoporins in differentiation was observed for gp210 in skeletal muscle differentiation (Gomez-Cavazos and Hetzer 2015). In these experiments, gp210 depletion led to endoplasmic reticulum stress suggested to be the primary catalyst for C2C12 myogenesis. It was hypothesized that components within the endoplasmic reticulum lumen, contiguous with the periplasmic space of the nuclear envelope, mediated cell fate commitment in response to physiological stress detected by the endoplasmic reticulum.
Overall, these studies demonstrate multiple modalities by which nucleoporins may contribute to development. Within this emerging area of research, studies of nucleoporins and their roles in reproductive development are a resurgent and exciting field. The new state of knowledge regarding noncanonical functions of nucleoporins in regulatory development provides unprecedented opportunities to study the role of nucleoporins in reproductive biology and physiology. Here, we highlight current knowledge regarding nucleoporin function in reproductive development in female and male animal models.
Roles of nucleoporins in gametogenesis
Gametogenesis is the main biological process in reproductive development, where diploid precursor cells differentiate and develop into mature haploid gametes with either an X or Y chromo-some (Spiller and Bowles 2015). In eukaryotes, gametogenesis is completed through meiosis, and proceeds into 2 different forms dependent on sex. In females, the production of ova is called oogenesis (Fig. 2), while the corresponding production of spermatozoa in males is known as spermatogenesis (Fig. 3) (Saitou and Miyauchi 2016; Spiller and Bowles 2015). The molecular mechanisms behind these processes and related meiotic disorders have been studied for several decades (Martin 2008), and the emergence of new technologies and conceptual advances facilitates characterization of novel aspects of gametogenic development.
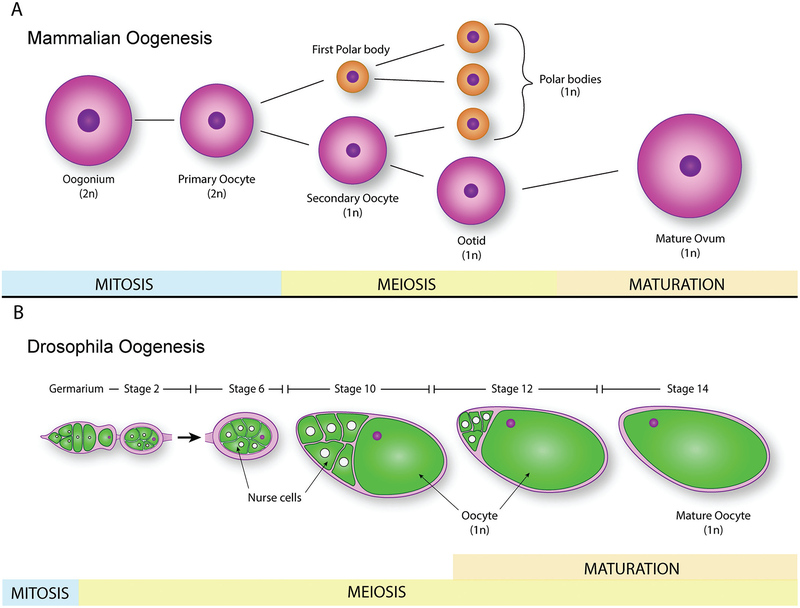
Fig. 2.
Mammalian and Drosophila oogenesis. (A) Schematic representation of mammalian oogenesis from oogonium to mature ovum, depicting mitosis, meiosis, and maturation stages. (B) Drosophila oogenesis schematic representation, where initiation of the oocyte maturation occurs during meiosis (depicted here as overlapping of maturation and meiotic stages). 2n, diploid cells; 1n, haploid cells.
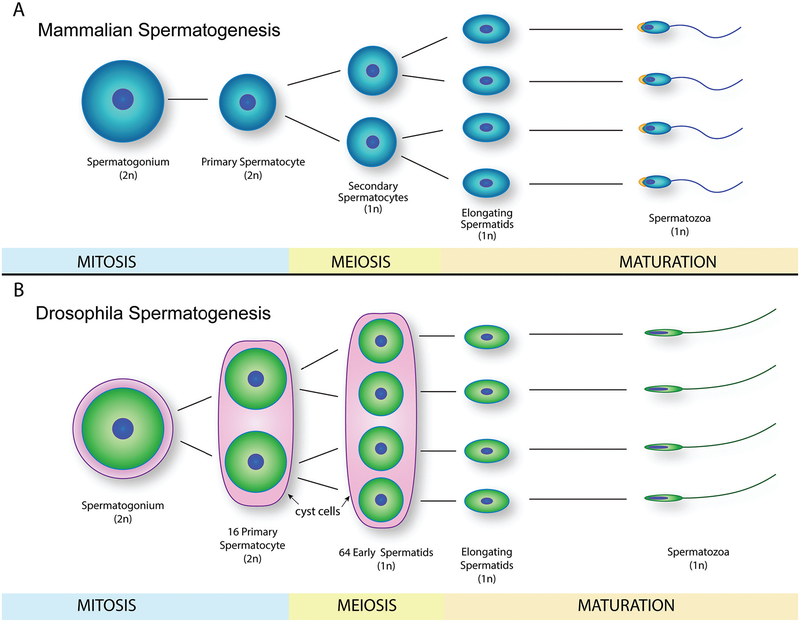
Fig. 3.
Mammalian and Drosophila spermatogenesis. (A) Schematic representation of mammalian spermatogenesis from spermatogonium to spermatozoa, depicting mitosis, meiosis, and maturation stages. (B) Drosophila spermatogenesis schematic representation, where spermatozoa have a more elongated nucleus and lack of acrosome region when compared with mammalian spermatozoa. 2n, diploid cells; 1n, haploid cells.
Nucleoporins and their association with reproductive development was first implicated by reports in various Drosophila and Xenopus models. Feldherr and colleagues suggested that NPC size determines macromolecular transport efficiency during oogenesis (Feldherr et al. 1998), while mutations in Nup154, the homo-logue of Nup155 in Drosophila, was reported to have negative impacts on Drosophila gametogenesis (Colozza et al. 2011; Gigliotti et al. 1998). Most recently, Nup50 was identified as a necessary factor for survival of primordial germ cells critical for both sexes (Park et al. 2016). Importantly, select nucleoporins have been observed to regulate gametogenesis and gonadal differentiation in a sex-specific manner, with a variety of molecular mechanisms underlying phenotype.
Nucleoporins in oogenesis
In most eukaryotes, oogenesis comprises 3 distinct sequential sub-processes: oocytogenesis, ootidogenesis, and maturation into an ovum (Fig. 2). Despite the overarching stepwise process in eukaryotes, mechanisms behind oogenesis vary among species, i.e., human and Drosophila oogenesis (Figs. 2A and 2B) (Avilés-Pagán and Orr-Weaver 2018; Nakamura et al. 2011). It is known that lifetime production of ova in most mammals is significantly smaller than in genera like Xenopus and Drosophila, where hundreds to thousands of eggs can be produced and released simultaneously.
Studies of Drosophila oogenesis (Fig. 2B) provided some of the first evidence of a role for nucleoporins in female gametogenesis. For example, Gigliotti and colleagues studied 2 mutant Nup154 alleles of lesser or greater severity, tlp1 and tlp2, respectively, and their roles in oogenesis and spermatogenesis (Gigliotti et al. 1998). Both mutations caused several abnormalities during gametogenesis, resulting in alterations of egg chambers, abnormal cell body morphology, and lack of chorionic dorsal appendages, all of which contributed to reproductive disorders that included incomplete sterility, diminished oocyte production, and decreased rates of fertilization (Gigliotti et al. 1998). Another study demonstrated that Nup154 mutation in oogenesis exhibited abnormal sub-cortical F-actin organization in mutated egg chambers during early stages of Drosophila oogenesis, along with alterations in late stage initiation of apoptosis (Riparbelli et al. 2007). This is in line with the understanding of actin as an essential factor for maturing oocytes (Clarke 2017; Mogessie and Schuh 2017).
Changes in NPC size as well as rate and quality of macromolecular nuclear transport occurs in oocytes during various stages of development (Feldherr et al. 1998). These alterations were linked to a key nuclear transport factor, p10, that exhibited elevated concentrations in early stage oocytes compared with mature oocytes, which were suggested to account for NPC size dependent differences in transport (Feldherr et al. 1998). It is important to note that p10 serves as a translocating factor that binds discretely to p62 nucleoporin (Nup62) as well as to the central channel complex containing p62, Nup58, and Nup54 on the cytoplasmic region of the NPC (Lane et al. 2000).
The nucleoporin Seh1, and its interactions with missing oocyte (Mio), was identified as critical to securing and maintaining normal meiosis and oocyte fate during Drosophila oogenesis (Senger et al. 2011). It was demonstrated that Mio and Seh1, a component of the Nup107-containing Y complex that is an essential subcomponent of the NPC (Hayashi et al. 2016), were crucial in the development of mature ovum, but were expendable for somatic development.
Nucleoporins in spermatogenesis
Spermatogenesis produces mature spermatozoa from spermatogenic germ cells by mitotic and meiotic divisions, and can be divided into distinct subprocesses: spermatocytogenesis, spermatidogenesis, and spermiogenesis (Fig. 3). However, in comparison with oogenesis, the interspecies differences in spermatogenesis are less apparent, as depicted in a comparison between human and Drosophila spermatogenesis (Figs. 3A and 3B).
Dynamics of the NPC are believed to play a role in later stages of sperm maturation and (or) fertilization, based on the observation of a global redistribution of NPCs to the redundant nuclear envelope compartment in developing spermatids in a mouse model (Ho 2010). At the level of the individual nucleoporins, a subset have been associated with spermatogenic disorders, with strong testis-specific mRNA and (or) protein enrichment (Cho et al. 2009; Whiley et al. 2012). The results from a study by Gigliotti and colleagues on Nup154 mutation in Drosophila identified severe spermatogenic defects compared with effects on oogenesis (Gigliotti et al. 1998), where complete sterility, greatly diminished testis size, and developmental arrest of spermatocytes was observed. In another Drosophila model of male meiosis, the Y subcomplex, composed of Nup37, Nup43, Nup85, Nup96, Nup107, Nup133, Nup160, Sec13, Seh1, and Elys, regulated nuclear lamin integrity essential for meiotic cytokinesis (Hayashi et al. 2016). Disruptions of other nucleoporins have also been reported to have consequences on sperm development. Tmem48/Ndc1 is a major nucleoporin that mediates interaction of the NPC with the nuclear membrane (Akiyama et al. 2013). Recently, the interaction between Tmem48/ Ndc1 and Sept12 was observed to be essential for mammalian spermiogenesis (Lai et al. 2016). In this work, Ndc1 demonstrates a capacity to regulate distribution of Sept12 that regulates sperm head and tail development.
The essential contribution of proper nucleoporin function to gametogenesis thus manifests in sex-specific manners. Understanding the diverse roles of nucleoporin function at the molecular level is critical for translational interpretation, as nonhuman models of gametogenesis may not precisely recapitulate human mechanisms. In spite of these limitations, the combination of patient cohorts exhibiting reproductive disorders paired with advanced high throughput sequencing technology and bioinformatic tools for deep data mining provides a powerful alternative to investigate the potential function of nucleoporins in human reproduction.
Translational relevance
As described earlier in this review, the Y subcomplex is a key feature of the NPC scaffold whose individual components have been associated with reproductive disorders in the clinic as well as in models at the bench. For example, independent work identified a missense D447N mutation in Nup107 in 4 female patients diagnosed with XX-gonadal dysgenesis who were members of an extended consanguineous family (Weinberg-Shukron et al. 2015). XX-ovarian dysgenesis is an uncommon disorder in humans characterized by nonfunctional streak ovaries and, when modeled in Drosophila, the authors observed outcomes that included abnormal oogenesis with structurally abnormal egg shells and egg chambers that resulted in almost complete infertility (Weinberg-Shukron et al. 2015).
Beyond gametogenesis, nucleoporins may be associated with human implantation. In one study, complete colocalization of Nup153 and Nup62, and partial colocalization of Tpr with nucleolar channel systems were observed (Guffanti et al. 2008). The nucleolar channel system is a well-characterized and transient feature of postovulation endometrium (Kittur et al. 2007), and the study by Guffanti et al. was the first demonstration of nucleoporin localization to the nucleolar channel system. The notion that nucleoporin-containing nucleolar channel systemss are key markers and possible contributors to proper implantation and uterine receptivity is supported by the results of a prospective study that discovered enrichment of FG nucleoporins following controlled ovarian hyperstimulation (Zapantis et al. 2013).
These examples of essential and constitutive roles for nucleoporins in gametogenesis (Table 1) establish a precedence for other regulatory functions for nucleoporins in reproductive development. With the increased recognition of individual nucleoporins able to regulate gene expression via differential genome binding and transcriptome remodeling (Pascual-Garcia et al. 2017), the use of advanced next generation sequencing tools can achieve greater depths of analysis and investigation into the systems-level role that nucleoporins may play in reproductive health.
Table 1.
Nucleoporin-associated alterations and disorders in male and female reproduction.
| Nucleoporin | Alteration | Model | Associated alterations in reproduction | Reference |
|---|---|---|---|---|
| AAAS | Aaas−/− | Mice | Female sterility with alterations in meiosis, diminished quality of mature oocytes, low fertility rates, and halted development after fertilization. | Carvalhal et al. 2017 |
| Elys | X-linked CG14215, Elys mutant strains | Drosophila | Female sterility, alterations in zygote viability with disruption of mitotic progression. | Hirai et al. 2018 |
| Nup50 | Nup50−/− | Mice | Impaired development of primordial germ cells in both males and females. | Park et al. 2016 |
| Nup107 | Missense D447N mutation | Human and Drosophila | Human XX-gonadal dysgenesis; in Drosophila model, females had abnormalities in egg shells, egg chambers, nurse cells, accompanied by cell death and sterility. | Weinberg-Shukron et al. 2015 |
| Nup107-Nup160 complex | UAS-Nup107 RNAi | Drosophila | Abnormality in nuclear lamina in male spermatocytes with impaired meiosis and cytokinesis. | Hayashi et al. 2016 |
| Nup154 | P-element induced mutation l(2)01501: tlp1 and tlp2 | Drosophila | Females had alterations in egg chamber development, low oocyte production, and decreased rates of fertilization. In males, alterations in cyst formation, spermatocyte proliferation, and meiotic progression with developmental arrest of spermatocytes. | Gigliotti et al. 1998 |
| Nup154 | tlp1 mutant | Drosophila | Females had abnormal organization of actin filaments egg chambers with defective nurse cell dumping and delayed apoptosis. | Riparbelli et al. 2007 |
| Nup154 | tlp1 mutant | Drosophila | Males exhibited increased apoptosis and reduced proliferation of germ cells, as well as alteration in meiosis and differentiation during spermatogenesis. | Colozza et al. 2011 |
| Ndc1/TMEM48 | Exon-trapped Tmem48 | Mice | TMEM48-associated spermatogenesis arrest at prophase ofmeiosis I. | Akiyama et al. 2013 |
| Ndc1 | SEPT12D197N mutation | Mice | Ndc1 modulates SEPT12 localization in male germ cell line. SEPT12-Ndc1-complex associated alterations during sperm head and tail formation. | Lai et al. 2016 |
| Seh1 | Seh1A15, Seh1A86 deletions | Drosophila | Drosophila females had diminished egg chambers, oocyte production, and lower hatching rate. Interaction of Seh1 with Mio is crucial for the development of mature ovum. | Senger et al. 2011 |
Note: AAAS, aladin WD repeat nucleoporin; Elys, embryonic large molecule derived from yolk sac; Mio, missing oocyte; Ndc1, Ndc1 transmembrane protein; Nup, nucleoporin; TMEM48, transmembrane protein 48; tlp, tulipano mutation; SEPT12, septin 12.
Leveraging high throughput technologies to address reproductive disorders
Next generation sequencing accelerates current understanding of global molecular dynamics underlying development (Faustino et al. 2008; Preston et al. 2018), and has emerged as an invaluable and versatile tool with the ability to identify rare genetic changes (Smith et al. 2007). Application of this technology is valuable for basic and clinical research and provides unique insights into system wide epigenomic and (or) transcriptome remodeling that occurs under normal and diseased conditions (Ernst et al. 2017; Wyles et al. 2015; Zhang et al. 2017).
These techniques have provided the foundation to design novel, robust, and sensitive cost-effective tools for clinical diagnosis. For example, preimplantation genetic screening leverages the advantage of next generation sequencing and bioinformatic analysis to enhance precision at reduced overhead (Fiorentino et al. 2014). Another important clinical use has been screening patients for novel sources of infertility (Bramble et al. 2016). In this study, whole exome sequencing was applied to interrogate the genome of a patient with primary ovarian failure and infertility. A loss-of-function N408Y mutation in the follicle stimulating hormone (FSH) receptor that rendered the patient insensitive to FSH stimulation was identified by this approach. Another application of whole exome sequencing helped identify gene candidate variants and potential underlying mechanisms afflicting an otherwise healthy 34-year-old woman who experienced 18 consecutive miscarriages (Filges et al. 2015). Comprehensive whole genome se quencing was used to interrogate the prevalence of de novo gametogenic mutations in 816 parent–offspring trios that defined parent-of-origin specific molecular signatures, as well as identified differing mutational mechanisms for spermatogenesis versus oogenesis (Goldmann et al. 2016). In an impressive demonstration of next generation sequencing at high resolution, single cell transcriptomes were analyzed to map developmental trajectories of individual human fetal germ cells and their gonadal niche cells (Li et al. 2017). These powerful sequencing approaches generate deep multi-dimensional data sets in basic and clinical research that must be parsed using bioinformatics (Hawkins et al. 2010). The availability of a variety of open source in silico tools makes such investigation accessible to researchers, which can be used to identify trends in genome dynamics and transcriptome remodeling underlying normal and dysfunctional reproductive development.
Indeed, methodologies that characterize global epigenomic or transcriptomic remodeling have identified noncanonical epigenomic regulatory functions for various nucleoporins in multiple model systems. Pioneering high throughput and chromatin immunoprecipitation studies in Drosophila established the precedence for regulatory chromatin binding and gene expression roles for Sec13 and Nup98, both of which activate specific gene subsets in a developmentally regulated manner, independent of the NPC and the nuclear envelope (Capelson et al. 2010). In support of this functional capacity for Nup98, genomic, transcriptomic, and proteomic work investigating epigenomic regulation of hematopoiesis identified Nup98 as critical for hematopoietic histone recruitment (Franks et al. 2017). In another example, high throughput gene expression profiling studies from Raices et al. complemented and corroborated earlier proteomic work by Hansson et al. showing that Nup210 was critical for pluripotency and myogenesis (Hansson et al. 2012; Raices et al. 2017). Epigenomic roles for nucleoporins have been implicated for cardio-genesis, through RNA-seq and network biology analyses applied to a Nup155-compromised stem cell line that gives rise to rhythmically impaired cardiomyocytes (Preston et al. 2018). In this study, the authors demonstrated that dysregulated gene expression with reprioritization of multiple functional ontologies occurred downstream of Nup155 disruption, with impairment of proper calcium handling and potential impacts on elements of the cardiac conduction system. Thus, these high throughput investigations are essential in defining the global impact of nucleoporins, and are relevant when consideration is given to the recognized systems-level functions of nucleoporins on development and disease (Capelson and Hetzer 2009).
Extant chromatin occupancy work has linked nucleoporin biology to chromatin wide-silencing and super enhancer access in the context of stem cell fate and identity (Ibarra et al. 2016; Ruben et al. 2011). Insights into this functional modality for nucleoporins in the context of reproductive physiology was first provided by early studies that observed specific interactions of Nup153 with the Drosophila genome (Vaquerizas et al. 2010). These nucleoporin-DNA areas were termed “Nucleoporin-Associated Regions”, or NARs, that regulated X-chromosome dosage compensation. Most recently, clinical studies linking mutant Nup107 to ovarian development demonstrates that other nucleoporins may be involved in reproductive development (Ren et al. 2018; Weinberg-Shukron et al. 2015), and are clear examples of exciting and emerging data in the nascent field of nucleoporin-regulated reproductive development.
Summary and future directions
The precedence for human reproduction to be regulated by discrete nucleoporins independent of their role in nucleocytoplasmic transport is supported by recent clinical evidence of nucleoporin mutations as the underlying cause for dysfunctional gonadogenesis. It is possible that nucleoporins possess sexually dimorphic phenotypes in this regard, with specific nucleoporins preferentially or exclusively contributing to proper oogenesis or spermatogenesis. Furthermore, the capacity to capture the underlying molecular etiology of reproductive disorders that arise from nucleoporin mutation is possible with a depth and precision facilitated by advanced next generation sequencing technologies. In the future, applications of these technologies in the clinical setting, with nucleoporins as an alternative marker/target to guide diagnosis, treatment, and resolution of abnormal gametogenesis and (or) gonadogenesis, may offer refined and personalized approaches to address reproductive disorders and infertility.
Acknowledgements
This work was supported by Sanford Research Funds and the Center for Pediatric Research (NIH Grant P20GM103620).
Footnotes
This review is part of a Special Issue entitled “Made in Canada”.
Contributor Information
Claudia C. Preston, Genetics and Genomics, Sanford Research, 2301 E. 60th Street N., Sioux Falls, SD 57104, USA.,
Emily C. Storm, Genetics and Genomics, Sanford Research, 2301 E. 60th Street N., Sioux Falls, SD 57104, USA.,
Riley J. Leonard, Genetics and Genomics, Sanford Research, 2301 E. 60th Street N., Sioux Falls, SD 57104, USA.
Randolph S. Faustino, Genetics and Genomics, Sanford Research, 2301 E. 60th Street N., Sioux Falls, SD 57104, USA; Department of Pediatrics, Sanford School of Medicine of the University of South Dakota, 1400 W. 22nd Street, Sioux Falls, SD 57105, USA.
References
- Akiyama K, Noguchi J, Hirose M, Kajita S, Katayama K, Khalaj M, et al. 2013. A mutation in the nuclear pore complex gene Tmem48 causes gameto-genesis defects in skeletal fusions with sterility (sks) mice. J. Biol. Chem 288(44): 31830–31841. doi: 10.1074/jbc.M113.492306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilés-Pagán EE, and Orr-Weaver TL 2018. Activating embryonic development in Drosophila. Semin. Cell Dev. Biol 84: 100–110. doi: 10.1016/j.semcdb.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, and Hurt E 2017. The nuclear pore complex: understanding its function through structural insight. Nat. Rev. Mol. Cell Biol 18(2): 73–89. doi: 10.1038/nrm.2016.147. [DOI] [PubMed] [Google Scholar]
- Bramble MS, Goldstein EH, Lipson A, Ngun T, Eskin A, Gosschalk JE, et al. 2016. A novel follicle-stimulating hormone receptor mutation causing primary ovarian failure: a fertility application of whole exome sequencing. Hum. Reprod 31(4): 905–914. doi: 10.1093/humrep/dew025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelson M, and Hetzer MW 2009. The role of nuclear pores in gene regulation, development and disease. EMBO Rep. 10(7): 697–705. doi: 10.1038/embor.2009.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelson M, Liang Y, Schulte R, Mair W, Wagner U, and Hetzer MW 2010. Chromatin-bound nuclear pore components regulate gene expression in higher eukaryotes. Cell, 140(3): 372–383. doi: 10.1016/j.cell.2009.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalhal S, Stevense M, Koehler K, Naumann R, Huebner A, Jessberger R, et al. 2017. ALADIN is required for the production of fertile mouse oocytes. Mol. Biol. Cell 28(19): 2470–2478. doi: 10.1091/mbc.E16-03-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho AR, Yang KJ, Bae Y, Bahk YY, Kim E, Lee H, et al. 2009. Tissue-specific expression and subcellular localization of ALADIN, the absence of which causes human triple A syndrome. Exp. Mol. Med 41(6): 381–386. doi: 10.3858/emm.2009.41.6.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HJ 2017. Actin brings chromosomes up to speed during oocyte maturation. Biol. Reprod 97(4): 519–521. doi: 10.1093/biolre/iox116. [DOI] [PubMed] [Google Scholar]
- Colozza G, Montembault E, Quénerch’du E, Riparbelli MG, D’Avino PP, and Callaini G 2011. Drosophila nucleoporin Nup154 controls cell viability, proliferation and nuclear accumulation of Mad transcription factor. Tissue Cell, 43(4): 254–261. doi: 10.1016/j.tice.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Ding JH, Xu X, Yang D, Chu PH, Dalton ND, Ye Z, et al. 2004. Dilated cardiomyopathy caused by tissue-specific ablation of SC35 in the heart. EMBO J. 23(4): 885–896. doi: 10.1038/sj.emboj.7600054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst EH, Grøndahl ML, Grund S, Hardy K, Heuck A, Sunde L, et al. 2017. Dormancy and activation of human oocytes from primordial and primary follicles: molecular clues to oocyte regulation. Hum. Reprod 32(8): 1684– 1700. doi: 10.1093/humrep/dex238. [DOI] [PubMed] [Google Scholar]
- Faustino RS, Behfar A, Perez-Terzic C, and Terzic A 2008. Genomic chart guiding embryonic stem cell cardiopoiesis. Genome Biol. 9(1): R6. doi: 10.1186/gb-2008-9-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldherr C, Akin D, and Moore MS 1998. The nuclear import factor p10 regulates the functional size of the nuclear pore complex during oogenesis. J. Cell Sci. 111(Pt. 13): 1889–1896. [DOI] [PubMed] [Google Scholar]
- Filges I, Manokhina I, Peñaherrera MS, McFadden DE, Louie K, Nosova E, et al. 2015. Recurrent triploidy due to a failure to complete maternal meiosis II: whole-exome sequencing reveals candidate variants. Mol. Hum. Reprod 21(4): 339–346. doi: 10.1093/molehr/gau112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino F, Biricik A, Bono S, Spizzichino L, Cotroneo E, Cottone G, et al. 2014. Development and validation of a next-generation sequencing-based protocol for 24-chromosome aneuploidy screening of embryos. Fertil. Steril 101(5): 1375–1382.e2. doi: 10.1016/j.fertnstert.2014.01.051. [DOI] [PubMed] [Google Scholar]
- Franks TM, McCloskey A, Shokirev MN, Benner C, Rathore A, and Hetzer MW 2017. Nup98 recruits the Wdr82-Set1A/COMPASS complex to promoters to regulate H3K4 trimethylation in hematopoietic progenitor cells. Genes Dev. 31(22): 2222–2234. doi: 10.1101/gad.306753.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliotti S, Callaini G, Andone S, Riparbelli MG, Pernas-Alonso R,Hoffmann G, et al. 1998. Nup154, a new Drosophila gene essential for male and female gametogenesis, is related to the nup155 vertebrate nucleoporin gene. J. Cell Biol. 142(5): 1195–1207. doi: 10.1083/jcb.142.5.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann JM, Wong WS, Pinelli M, Farrah T, Bodian D, Stittrich AB, et al. 2016. Parent-of-origin-specific signatures of de novo mutations. Nat. Genet 48(8): 935–939. doi: 10.1038/ng.359. [DOI] [PubMed] [Google Scholar]
- Gomez-Cavazos JS, and Hetzer MW 2015. The nucleoporin gp210/Nup210 controls muscle differentiation by regulating nuclear envelope/ER homeostasis. J. Cell Biol 208(6): 671–681. doi: 10.1083/jcb.201410047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti E, Kittur N, Brodt ZN, Polotsky AJ, Kuokkanen SM, Heller DS, et al. 2008. Nuclear pore complex proteins mark the implantation window in human endometrium. J. Cell Sci 121(Pt. 12): 2037–2045. doi: 10.1242/jcs.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson J, Rafiee MR, Reiland S, Polo JM, Gehring J, Okawa S, et al. 2012. Highly coordinated proteome dynamics during reprogramming of somatic cells to pluripotency. Cell Rep. 2(6): 1579–1592. doi: 10.1016/j.celrep.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RD, Hon GC, and Ren B 2010. Next-generation genomics: an integrative approach. Nat. Rev. Genet 11(7): 476–486. doi: 10.1038/nrg2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi D, Tanabe K, Katsube H, and Inoue YH 2016. B-type nuclear lamin and the nuclear pore complex Nup107–160 influences maintenance of the spindle envelope required for cytokinesis in Drosophila male meiosis. Biol. Open, 5(8): 1011–1021. doi: 10.1242/bio.017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K, Wang Z, Miura K, Hayashi T, Awasaki T, Wada M, et al. 2018. Genetic Analyses of Elys Mutations in Drosophila Show Maternal-Effect Lethality and Interactions with Nucleoporin Genes. G3 (Bethesda), 8(7): 2421–2431. doi: 10.1534/g3.118.200361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HC 2010. Redistribution of nuclear pores during formation of the redundant nuclear envelope in mouse spermatids. J. Anat 216(4): 525–532. doi: 10.1111/j.1469-7580.2009.01204.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra A, Benner C, Tyagi S, Cool J, and Hetzer MW 2016. Nucleoporin-mediated regulation of cell identity genes. Genes Dev. 30(20): 2253–2258. doi: 10.1101/gad.287417.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto FV, Benner C, and Hetzer MW 2015. The nucleoporin Nup153 regulates embryonic stem cell pluripotency through gene silencing. Genes Dev. 29(12): 1224–1238. doi: 10.1101/gad.260919.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittur N, Zapantis G, Aubuchon M, Santoro N, Bazett-Jones DP, and Meier UT 2007. The nucleolar channel system of human endometrium is related to endoplasmic reticulum and R-rings. Mol. Biol. Cell, 18(6): 2296– 2304. doi: 10.1091/mbc.e07-02-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai TH, Wu YY, Wang YY, Chen MF, Wang P, Chen TM, et al. 2016. SEPT12-NDC1 complexes are required for mammalian spermiogenesis. Int. J. Mol. Sci 17(11): 1911. doi: 10.3390/ijms17111911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane CM, Cushman I, and Moore MS 2000. Selective disruption of nuclear import by a functional mutant nuclear transport carrier. J. Cell Biol. 151(2): 321–332. doi: 10.1083/jcb.151.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Dong J, Yan L, Yong J, Liu X, Hu Y, et al. 2017. Single-cell RNA-Seq analysis maps development of human germline cells and gonadal niche interactions. Cell Stem Cell, 20(6): 858–873.e4. doi: 10.1016/j.stem.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Martin RH 2008. Meiotic errors in human oogenesis and spermatogenesis. Reprod. Biomed. Online, 16(4): 523–531. doi: 10.1016/S1472-6483(10)60459-2. [DOI] [PubMed] [Google Scholar]
- Mogessie B, and Schuh M 2017. Actin protects mammalian eggs against chromosome segregation errors. Science, 357(6353): eaal1647. doi: 10.1126/science.aal1647. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kobayashi K, Nishimura T, and Tanaka M 2011. Ovarian germ-line stem cells in the teleost fish, medaka (Oryzias latipes). Int. J. Biol. Sci 7(4): 403–409. doi: 10.7150/ijbs.7.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Lee B, Clurman BE, and Lee K 2016. NUP50 is necessary for the survival of primordial germ cells in mouse embryos. Reproduction, 151(1): 51–58. doi: 10.1530/REP-14-0649. [DOI] [PubMed] [Google Scholar]
- Pascual-Garcia P, Debo B, Aleman JR, Talamas JA, Lan Y, Nguyen NH, et al. 2017. Metazoan nuclear pores provide a scaffold for poised genes and mediate induced enhancer-promoter contacts. Mol. Cell, 66(1): 63–76.e6. doi: 10.1016/j.molcel.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popken J, Schmid VJ, Strauss A, Guengoer T, Wolf E, and Zakhartchenko V 2016. Stage-dependent remodeling of the nuclear envelope and lamina during rabbit early embryonic development. J. Reprod. Dev 62(2): 127–135. doi: 10.1262/jrd.2015-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston CC, Wyles SP, Reyes S, Storm EC, Eckloff BW, and Faustino RS 2018. NUP155 insufficiency recalibrates a pluripotent transcriptome with network remodeling of a cardiogenic signaling module. BMC Syst. Biol 12(1):62. doi: 10.1186/s12918-018-0590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raices M, and D’Angelo MA 2012. Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions. Nat. Rev. Mol. Cell. Biol 13(11): 687–699. doi: 10.1038/nrm3461. [DOI] [PubMed] [Google Scholar]
- Raices M, Bukata L, Sakuma S, Borlido J, Hernandez LS, Hart DO, and D’Angelo MA 2017. Nuclear pores regulate muscle development and maintenance by assembling a localized Mef2C complex. Dev. Cell, 41(5): 540– 554.e7. doi: 10.1016/j.devcel.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Diao F, Katari S, Yatsenko S, Jiang H, Wood-Trageser MA, and Rajkovic A 2018. Functional study of a novel missense single-nucleotide variant of NUP107 in two daughters of Mexican origin with premature ovarian insufficiency. Mol. Genet. Genomic Med 6(2): 276–281. doi: 10.1002/mgg3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riparbelli MG, Gigliotti S, and Callaini G 2007. The Drosophila nucleoporin gene nup154 is required for correct microfilament dynamics and cell death during oogenesis. Cell Motil. Cytoskeleton, 64(8): 590–604. doi: 10.1002/cm.20206. [DOI] [PubMed] [Google Scholar]
- Ritterhoff T, Das H, Hofhaus G, Schröder RR, Flotho A, and Melchior F 2016. The RanBP2/RanGAP1*SUMO1/Ubc9 SUMO E3 ligase is a disassembly machine for Crm1-dependent nuclear export complexes. Nat. Commun 7: 11482. doi: 10.1038/ncomms11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben GJ, Kirkland JG, MacDonough T, Chen M, Dubey RN, Gartenberg MR, and Kamakaka RT 2011. Nucleoporin mediated nuclear positioning and silencing of HMR. PLoS One, 6(7): e21923. doi: 10.1371/journal.pone.0021923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, and Miyauchi H 2016. Gametogenesis from pluripotent stem cells. Cell Stem Cell, 18(6): 721–735. doi: 10.1016/j.stem.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Senger S, Csokmay J, Akbar T, Jones TI, Sengupta P, and Lilly MA 2011. The nucleoporin Seh1 forms a complex with Mio and serves an essential tissue-specific function in Drosophila oogenesis. Development, 138(10): 2133– 2142. doi: 10.1242/dev.057372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MG, Gianoulis TA, Pukatzki S, Mekalanos JJ, Ornston LN, Gerstein M, and Snyder M 2007. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 21(5): 601–614. doi: 10.1101/gad.1510307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller CM, and Bowles J 2015. Sex determination in mammalian germ cells. Asian J. Androl 17(3): 427–432. doi: 10.4103/1008-682X.150037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquerizas JM, Suyama R, Kind J, Miura K, Luscombe NM, and Akhtar A 2010. Nuclear pore proteins nup153 and megator define transcriptionally active regions in the Drosophila genome. PLoS Genet. 6(2): e1000846. doi: 10.1371/journal.pgen.1000846.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Appen A, and Beck M 2016. Structure determination of the nuclear pore complex with three-dimensional cryo electron microscopy. J. Mol. Biol 428(10 Pt. A): 2001–2010. doi: 10.1016/j.jmb.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg-Shukron A, Renbaum P, Kalifa R, Zeligson S, Ben-Neriah Z, Dreifuss A, et al. 2015. A mutation in the nucleoporin-107 gene causes XX gonadal dysgenesis. J. Clin. Invest 125(11): 4295–4304. doi: 10.1172/JCI83553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiley PA, Miyamoto Y, McLachlan RI, Jans DA, and Loveland KL 2012. Changing subcellular localization of nuclear transport factors during human spermatogenesis. Int. J. Androl 35(2): 158–169. doi: 10.1111/j.1365-2605.2011.01202.x. [DOI] [PubMed] [Google Scholar]
- Wyles SP, Faustino RS, Li X, Terzic A, and Nelson TJ 2015. Systems-based technologies in profiling the stem cell molecular framework for cardioregenerative medicine. Stem Cell Rev 11(3): 501–510. doi: 10.1007/s12015-014-9557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaseen NR, and Blobel G 1999. GTP hydrolysis links initiation and termination of nuclear import on the nucleoporin nup358. J. Biol. Chem 274(37): 26493–26502. doi: 10.1074/jbc.274.37.26493. [DOI] [PubMed] [Google Scholar]
- Zapantis G, Szmyga MJ, Rybak EA, and Meier UT 2013. Premature formation of nucleolar channel systems indicates advanced endometrial maturation following controlled ovarian hyperstimulation. Hum. Reprod 28(12): 3292–3300. doi: 10.1093/humrep/det358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Chen L, Feng G, Xiang W, Zhang K, Chu M, and Wang P 2017. MicroRNA mediating networks in granulosa cells associated with ovarian follicular development. Biomed Res. Int 2017: 4585213. doi: 10.1155/2017/4585213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen S, Yoo S, Chakrabarti S, Zhang T, Ke T, et al. 2008. Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell, 135(6): 1017–1027. doi: 10.1016/j.cell.2008.10.022. [DOI] [PubMed] [Google Scholar]