Figure 7.
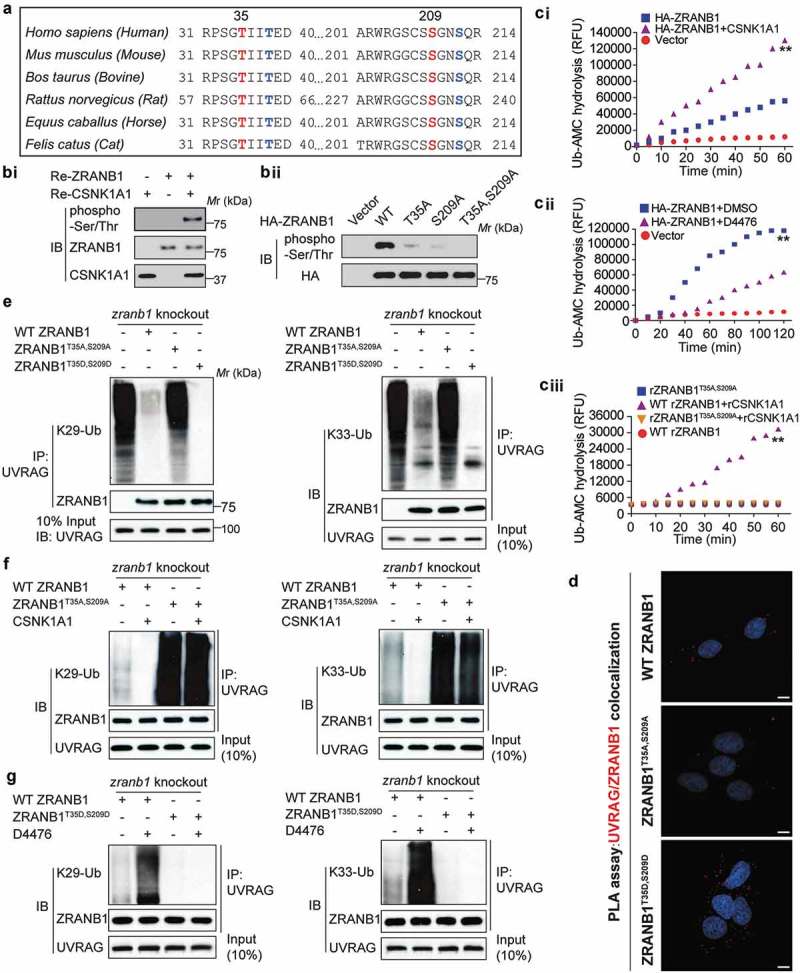
ZRANB1 is a CSNK1A1 substrate, and this phosphorylation activates the DUB activity of ZRANB1. (a) Putative CSNK1A1 phosphorylation sites predicted by Scansite within ZRANB1 orthologs. (bi) The phosphorylation of ZRANB1 was found using a phospho-Ser/Thr antibody. (bii) HEK293T cells were transfected with wild-type or mutant ZRANB1 constructs, then phosphorylation of ZRANB1 was detected using the phospho-Ser/Thr antibody. (ci and cii) CSNK1A1 activates ZRANB1 in cells. ZRANB1 was immunoprecipitated from HEK293T cells coexpressed with CSNK1A1 (ci) or treated with 10 μM D4476 (cii), and followed by Ub-AMC hydrolysis assay. (ciii) T35A S209A mutation blocks ZRANB1 activation by CSNK1A1. Recombinant ZRANB1 protein, T35A S209A mutation was mixed with or without CSNK1A1 in vitro, and Ub-AMC assay was performed. RFU represents relative fluorescence units. (d) Proximity ligation assay (PLA) was performed to confirm the interaction between UVRAG, ZRANB1 and their mutants. Scale bars: 10 µm. (e) zranb1−/− MEF cells were virally transfected with indicated plasmids, and the K29- and K33-ubiquitination of UVRAG was analyzed. (f) zranb1−/− MEF cells were virally transfected with indicated plasmids with or without CSNK1A1, and the K29- and K33-ubiquitination of UVRAG was analyzed. (g) zranb1−/− MEF cells were virally transfected with indicated plasmids with or without 10 μM D4476, and the K29- and K33-ubiquitination of UVRAG was analyzed.
