INTRODUCTION:
Over the past 20 years, aberrant crypt foci (ACF) have emerged as potential precursors and biomarkers for colorectal cancer (CRC). However, data regarding their molecular pathogenesis, as well as their endoscopic and histological identification, remain inconsistent.
METHODS:
A wide cohort of ACF from 100 control subjects and 100 case patients, including patients with adenoma and CRC, were characterized for endoscopic, morphologic, and molecular features.
RESULTS:
We observed that among all the endoscopic features evaluated, only the number of large ACF correlated with CRC risk (P = 0.003), whereas the histological classification, as assessed by 2 different pathologists, was inconsistent and did not differ between control and case patients. Moreover, only a few APC and BRAF mutations and no microsatellite instability were detected in our samples. KRAS mutations were detected in 16.3% of ACF samples, which also exhibited increased MGMT hypermethylation. However, none of those events were found to be predictive of CRC risk.
DISCUSSION:
Although ACF might be preneoplastic lesions of the colon, they are not suitable biomarkers for assessing CRC progression.
INTRODUCTION
Aberrant crypt foci (ACF) are the earliest visible lesions in the colorectum and are considered potential precursors of colorectal cancer (CRC). They are defined as crypts with altered luminal openings, thickened epithelium, and larger in size than normal crypts. In addition, although ACF can arise in both the proximal and distal colon, they are mostly observed in the distal colon and rectum (1). ACF were first detected in the colon of rodents treated with colon-specific carcinogens (2–4), being later identified in human patients at a high risk of CRC (5).
The association of the size and number of ACF with CRC risk in humans is somehow controversial, with studies for and against these findings (6–12). These lesions also exhibit dysplasia, an increased proliferative index and some genetic alterations such as KRAS, APC, and BRAF mutations, commonly observed in adenomas and carcinomas (13). Nevertheless, the frequency and distribution of these alterations vary substantially between studies and among CRC risk groups (6,14,15), complicating the clinical utility of ACF as CRC biomarkers.
The most widely accepted histological approach is to classify ACF as hyperplastic and dysplastic, as recommended by the World Health Organization (16). The frequency of dysplastic ACF is low (10,11,17,18); however, it seems to have potential for malignant degeneration (6,19–22). In addition, studies of hyperplastic ACF have suggested that these, too, might have malignant potential, albeit via the serrated pathway of carcinogenesis (23).
High-magnification chromoendoscopy (CE) permits the direct observation of ACF and allows for the identification of several features that have been correlated with ACF histology and CRC risk (6,19,23). In fact, the most common definition of ACF is based on endoscopy crypt patterns after staining with methylene blue. ACF are clusters of crypts that are stained darker than the surrounding mucosa, have larger diameters, often with oval or slit-like lumens and thicker epithelial linings (6). Several human studies have demonstrated that ACF can be identified and characterized by conventional and electronic CE using magnification and high-definition scopes (6,7,11,19,20,24). Nevertheless, the data reported to date remain inconsistent (25), and the rate of agreement between the presence of ACF and their histologic confirmation varies substantially (6,7), thus further hindering their utility as surrogate markers for CRC. Indeed, most ACF reportedly remain in a dormant state or even regress and disappear (26,27), and according to 1 multicenter study, rectal ACF were difficult to reidentify during follow-up examination (11).
Despite evidence supporting the notion that ACF are precancerous lesions, there are many inconsistences in the data regarding their molecular pathogenesis, as well as its endoscopic and histological identification. Therefore, the aim of this study was to determine whether rectal ACF are biomarkers of CRC risk by characterizing the endoscopic, morphologic, and molecular features of ACF samples collected from subjects without colonic lesions (controls), with adenoma and CRC.
METHODS
Subjects
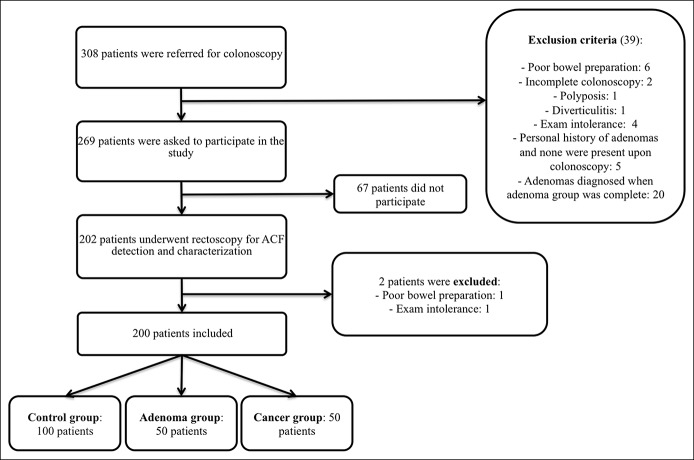
This study was approved by the Ethics Committee at the Hospital Clinic of Barcelona, and all participants provided their written consent. Individuals were prospectively recruited from the regular patient agenda of the Endoscopy Unit at the Hospital Clinic of Barcelona. During the inclusion period, colonoscopy reports in the Endoscopy Unit were reviewed daily. Individuals with a colonoscopy not reaching cecum (or ileocolonic anastomosis, if applicable) and/or with a poor bowel preparation in any colonic segment were excluded. Subjects were invited to participate in the study via phone call a few days after the colonoscopy. They were selected and divided into 2 groups based on their endoscopic findings: (i) Control group: individuals with a normal colonoscopy and without a personal history of adenomas or CRC (n = 100) and (ii) Case group: patients with a personal history of CRC or current CRC (n = 50) or patients with ≥1 current colonic adenomas (n = 50). Patient exclusion criteria are detailed in Figure 1. For ACF detection, a different examiner, who was blinded to each patient's study group, performed a rectoscopy. The interval between the colonoscopy and the rectoscopy was less than 1 month.
Figure 1.
Patients' flowchart.
Endoscopy assessment
A systematic examination of the distal 10 cm of the rectum was performed with a high-definition colonoscope (Olympus H180, Evis Exera II processor, Olympus Europe) in all patients. ACF were defined as crypts with a larger diameter than the normal mucosa, a thicker epithelial lining, and a dilated crypt lumen. ACF that raised >2 mm were considered polyps. The number of ACF per patient was categorized as less than 5, 5 to 15, or more than 15. The rectum was examined clockwise, proximal to distal, to record ACF features and their location, first with narrow-band imaging (NBI) and then with methylene blue 0.5% CE. CE was considered the gold standard for ACF detection. The size of ACF, as assessed by CE, was classified as small (<20 crypts per ACF), medium (20–40 crypts per ACF), or large (>40 crypts per ACF). The shape of the crypt lumens, as visualized by CE, was characterized as semicircular-oval, asteroid-like, or irregular. ACF vascular pattern intensity (VPI) was described as weak, normal, or strong in comparison with the appearance of the surrounding mucosa, as visualized by NBI.
Pathological assessment
Normal mucosa and the 5 largest ACF were biopsied for each patient. ACF were immediately immersed in tissue freezing medium (OCT) and stored at −80 °C, whereas the normal mucosa was conserved in PBS at −80 °C. The tissue sections were stained with hematoxylin and eosin and analyzed by light microscopy. All samples were evaluated twice over a period of 6 months by 2 different pathologists (A and B) who were blinded to the endoscopic classification and to each other's diagnoses. Discordant diagnoses were reviewed to reach a consensus diagnosis. Histological findings were classified as inadequate sample, normal mucosa, hyperplastic ACF, and dysplastic ACF, according to the WHO classification (16). The “serrated morphology” category was incorporated into our final diagnosis to evaluate the possible role of ACF in the serrated pathway.
Molecular analysis
For feasibility reasons, molecular characterization was only performed for the first 3 histologically confirmed ACF samples from each patient. DNA from ACF and normal mucosa was extracted using the All Prep DNA/RNA Mini Kit (Qiagen, Hilden, Germany) in accordance with the manufacturer's recommendations and quantified using a NanoDrop Spectrophotometer ND-1000 (Thermo Fisher Scientific, Waltham, MA).
KRAS mutational analysis
A fragment of the KRAS gene spanning codons 12 and 13 was amplified by COLD-PCR using the following primers: F, 5'-GCCTGCTGAAAATGACTGAA-3', and R, 5'-AGAATGGTCCTGCACCAGTAA-3'.
APC mutational analysis
APC mutations were analyzed from 2 amplified fragments (A and B) that spanned the majority (82.6%) of the APC gene mutations. Primer sequences were (i) A-F (5'-CAGTGAGAATACGTCCACACCT-3') and B-F (5'-TTTGAGAGTCGTTCGATTGC-3') and (ii) A-R (5'-CATTCCACTGCATGGTTCAC-3') and B-R (5'-TGATGACTTTGTTGGCATGG-3').
BRAF mutational analysis
BRAF V600E mutation genotyping was performed by Real-Time Taqman PCR using primers and probes designed by Applied Biosystems Custom Genotyping Assay Service.
Microsatellite instability analysis
The microsatellite instability (MSI) Analysis System, consisting of 5 nearly monomorphic mononucleotide markers (BAT-25, BAT-26, NR-21, NR-24, and MONO-27) and 2 polymorphic pentanucleotide markers (Penta C and Penta D) were used according to the manufacturer's guidelines (Promega, Wisconsin).
Methylation analysis
MGMT gene promoter methylation levels were investigated using pyrosequencing-based methylation analysis, as described previously (28).
Statistical analysis
CE and the consensus histological diagnoses were considered the gold standard for ACF detection and diagnostic confirmation, respectively. SPSS statistical software (IBM 2012, IBM SPSS Statistics, Version 20.0. Armonk, NY) was used for data analysis. Results for continuous variables were summarized using mean and SD or median and interquartile range for skewed data. Frequencies (%) were used to summarize categorical variables, and 95% confidence intervals were calculated when relevant. Student t or Mann-Whitney tests were used to compare the distribution of continuous variables by their outcome. Pearson χ2 or Fisher exact tests were used to test for any association between categorical variables and outcome. All analyses were exploratory, and 2-tailed tests with a significance level of 5% were used throughout. The association between endoscopic features and study group were adjusted by age and sex using binary logistic regression. Paired analyses were performed when comparing molecular changes in normal mucosa and ACF. Inter- and intra-pathologist concordance for histological diagnosis were calculated by Weighed k-statistics and defined as follows: fair, 0.21–0.40; moderate, 0.41–0.60; good, 0.61–0.80; and very good, 0.81–1.00.
RESULTS
Patient baseline characteristics
Two hundred patients (56% female, age 62.9 ± 13.8 years) were included in this study. The control group was composed of more females, of younger age, than the study group (66% vs 46%, P = 0.05 and 57.62 ± 15.58 years vs 68.15 ± 9.26 years, P < 0.001, respectively). Among the patients with adenoma, 29 (58%) had ≥1 advanced adenoma. In the CRC group, 22 patients (44%) had a previous CRC, and 28 (56%) had a current CRC. Inclusion and exclusion criteria are shown in Figure 1, and colonoscopy indications are detailed in Table S1 (see Supplementary Digital Content 1, http://links.lww.com/CTG/A46).
Endoscopic features of ACF
CE detected the presence of at least 1 ACF in 176/200 (88%) patients. Forty-three individuals (21.5%) exhibited less than 5 ACF, 94 (47.0%) had between 5 and 15 ACF, and 63 (31.5%) had more than 15 ACF. A total of 1,103 ACF were characterized by CE, whereas 768 were characterized by NBI. Size evaluation determined that 305 ACF (27.2%) were small, 366 (33.2%) were medium, and 432 (39.2%) were large. The shape of the crypt lumens was semicircular-oval in 80.1% of the ACF, asteroid-like in 10.1%, and irregular in 9.8%. Finally, ACF VPI was weak in 525 ACF (68.4%), normal in 220 ACF (28.6%), and strong in only 23 ACF (3%).
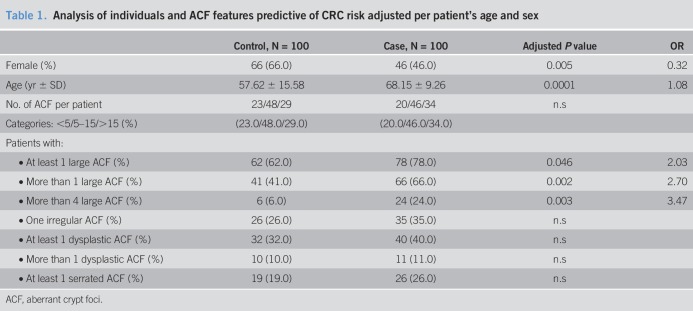
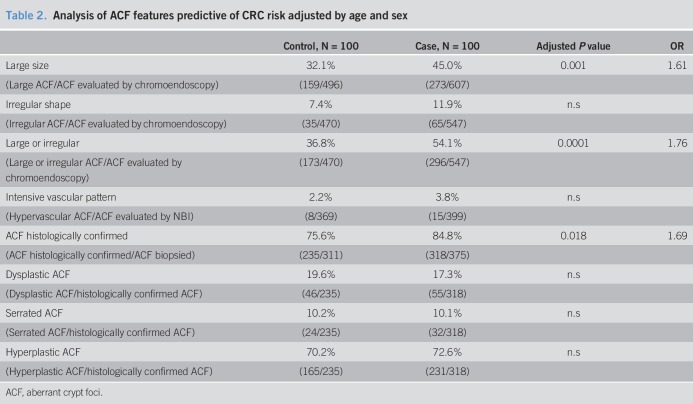
As is shown in Tables 1 and 2, only the presence of large ACF was related to the CRC risk group. In fact, the number of large ACF increased progressively toward CRC risk (control, 6.06%; adenoma, 20%; CCR, 28%). Conversely, neither the number of ACF per subject, the lumen morphology, nor VPI was related to CRC risk.
Table 1.
Analysis of individuals and ACF features predictive of CRC risk adjusted per patient's age and sex
Table 2.
Analysis of ACF features predictive of CRC risk adjusted by age and sex
ACF histology
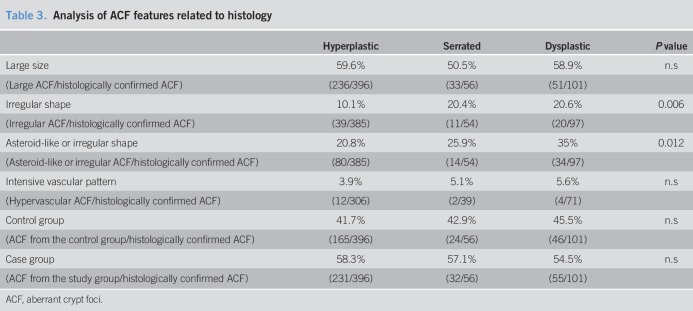
Although 686 ACF were detected endoscopically, only 553 were confirmed by histology (38 inadequate samples, 95 normal mucosa) (see Figure S1, Supplementary Digital Content 1, http://links.lww.com/CTG/A46). According to the consensus diagnosis, 553 ACF samples were classified as hyperplastic ACF (71.6%), serrated ACF (10.1%), or dysplastic ACF (18.3%) (Figure 2). Consequently, the diagnostic yield of endoscopy (histologically confirmed ACF/ACF detected with CE) was 80.6%. Pathologist A showed a weighed K for intraobserver concordance of 0.59, and pathologist B 0.71 (P ≤ 0.001). The best weighed K for interobserver concordance was only 0.25 (P < 0.001). Interestingly, the histology of ACF was not related to gender, sex, or CRC risk group (Table 2). Although dysplastic and serrated ACF exhibited an irregular shape more frequently than hyperplastic ACF, their size and VPI were not associated with ACF histology (Table 3).
Figure 2.
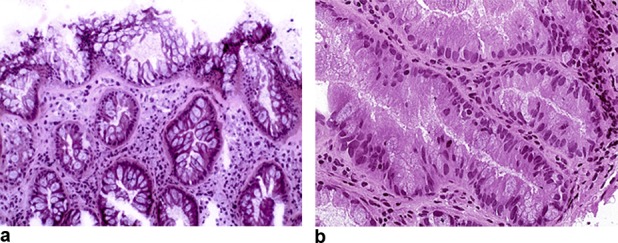
Histological sections of 2 aberrant crypt foci, with hyperplastic change, showing nondysplastic distorted architecture of the surface epithelium with widening and hyperplastic contour of the luminal end of the crypts (a) (H&E, ×100), and dysplastic change with hyperchromatic, cigar-shaped nuclei, pseudostratification, dense eosinophilic cytoplasm, mitotic figures, and loss of cytoplasmic mucin (b) (H&E, ×200).
Table 3.
Analysis of ACF features related to histology
Molecular analysis of ACF
The first 3 histologically confirmed ACF from each patient were used for molecular analysis, totaling 294 ACF samples. Of these, 128 were from 67 control subjects (47 female (70%); age 58.3 ± 15.2 years) and 166 were from 81 case patients, of which 40 were from patients with adenoma and 41 were from patients with CRC (32 female (39%); age 67.7 ± 9.9 years). Of this cohort of ACF samples, 146/294(49.7%) were categorized as large lesions. Histological examination revealed that 197 ACF (67.0%) were hyperplastic, 35 (12.0%) serrated, and 62 (21.0%) dysplastic lesions (see Figure S1, Supplementary Digital Content 1, http://links.lww.com/CTG/A46).
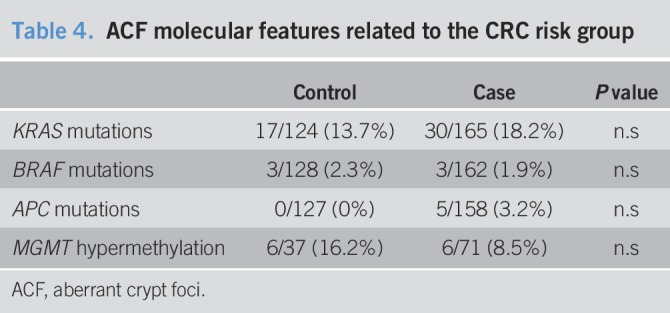
APC mutation and MSI analysis in ACF
APC mutations were determined for 285/294 (96.9%) ACF samples. The APC variant E1317Q was found in 5/285 (1.7%) samples (Table 4); however, these ACF belonged to patients who exhibited the same variant in their normal mucosa, indicating that it was germline and not related to ACF formation. MSI status was evaluated for 276/294 (94.2%) ACF samples. All samples analyzed were MSS.
Table 4.
ACF molecular features related to the CRC risk group
BRAF and KRAS mutations in ACF
BRAF and KRAS mutations were examined in 290/294 (98.6%) and 289/294 (98.3%) ACF samples, respectively. The BRAF V600E mutation was detected in only 6/290 (2.1%) ACF samples, whereas KRAS was mutated in 48/289 (16.3%) ACF samples. Moreover, as described previously, no ACF sample simultaneously exhibited BRAF and KRAS mutations (29).
MGMT methylation status analysis in ACF
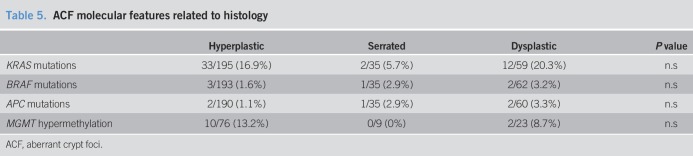
As KRAS mutations have been previously associated with the serrated alternative pathway of carcinogenesis (30), we determined the MGMT methylation status in 33 KRAS-mutated and 75 KRAS wild-type ACF samples, as well as in their surrounding normal mucosa. Overall, the level of methylation was significantly higher in ACF samples compared with their corresponding normal mucosa (5.54 ± 3.70 vs 4.23 ± 2.31, P = 0.004). However, there were no differences in the levels of methylation among the control and case groups or between those ACF samples with mutated or wild-type KRAS status (see Figure S2A, B and C, Supplementary Digital Content 1, http://links.lww.com/CTG/A46). Next, we analyzed the methylation results as a categorical variable using the mean levels in the normal mucosa methylation as the cutoff for ACF hypermethylation (mean ± 2 SD, which corresponded to 8.66%). Interestingly, by using this cutoff, we observed significantly more hypermethylated samples in the set of KRAS mutated ACF than in the set of ACF displaying wild-type KRAS (21% vs 5.71%, P = 0.02). Nevertheless, we did not detect any difference in the distribution of the hypermethylated ACF among the control and case groups or according to their histology (Tables 4 and 5).
Table 5.
ACF molecular features related to histology
DISCUSSION
The present case-control study uses a translational approach to evaluate the role of rectal ACF as a precursor lesion of CRC and its clinical application as an intermediate end point in CRC carcinogenesis. First, we performed in vivo and in situ detection and characterized ACF by advanced endoscopy in 100 individuals without colonic lesions and 100 patients at risk of CRC. Second, 2 different pathologists evaluated a set of 686 ACF samples twice to reach a consensus diagnosis on the basis of standardized criteria. Last, a molecular profile of common CRC alterations was assessed for these lesions. Our results showed that rectal ACF may be preneoplastic lesions and play a role in CRC carcinogenesis; however, they are not reliable biomarkers because their morphological and molecular findings were not consistent and there was no association with the risk group.
Previous studies have reported the diagnostic yield of endoscopy when using histology as gold standard to be between 60% and 68% (11,31). In our cohort, 80% of the lesions identified by endoscopy as potential ACF were corroborated by their histology, thus indicating that high-definition CE was a suitable technique for detecting these subtle lesions (32).
Previously, it has been reported that there is a stepwise increase in the number and prevalence of rectal ACF in relation to the CRC risk group (18,24). However, in accordance with data from multicenter studies (10,11), we did not find the number of ACF to be associated with CRC. On the other hand, although none of the other endoscopic features were predictive of CRC risk, we found that the number of large ACF was associated with the CRC risk group. Unfortunately, this endoscopic feature was not associated with a specific histopathological diagnosis or molecular pathway, rendering this observation of dubious clinical utility.
Previous data showed that dysplastic ACF were specially related to CRC risk (19). In addition, elongated and asteroid-like lumens are characteristic of dysplasia and hyperplasia, respectively (20,33). However, we only found irregular lumens to be associated with dysplastic and serrated ACF. This is in accordance with a previous study that showed that endoscopic features were inconsistent across endoscopists and did not accurately predict histology (31).
Histological characterization of ACF remains a challenge. There is significant variability across numerous studies in terms of the tissue sampling procedures and ACF classification (34,35). Two pathologists examined all specimens, twice, in random order, yet neither the interobserver nor the intraobserver concordances were satisfactory. Considering that we are evaluating very subtle changes, the small size of the samples (average of 2 mm) and the lack of orientation during sampling may have influenced these poor results. Nonetheless, this limitation reflects the difficulties in classifying ACF morphologically and suggests the necessity of a more careful and objective standardization among pathologists, such as examining different ACF sections to accurately assess dysplasia in these lesions, which may be focally represented. Our cohort of ACF samples was mainly composed of hyperplastic lesions, accounting for only 18.5% of dysplastic ACF, which is in agreement with the previously reported low prevalence of dysplasia. In fact, some studies did not even detect dysplastic ACF (10,17). Furthermore, as the appearance of sessile serrated polyps has been mainly associated with the proximal colon (36,37), we were only able to detect a modest percentage of serrated ACF. Nevertheless, we did not find any histological category to be predictive of CRC risk.
Several previous studies have stated that ACF harbor genetic alterations that might lead to malignant transformation, but these data remain controversial (14,15,38). APC mutations are considered an early event in CRC carcinogenesis; however, several studies have reported that APC mutations are found in ACF lesions from patients with familial adenomatous polyposis, whereas they are infrequent in sporadic ACF (15,38,39). Similarly, in our cohort of ACF samples, we found APC alterations to be rare. In addition, although mutations in BRAF are commonly associated with serrated lesions from the proximal colon, including ACF (40–42), others have detected them in only 2% of sporadic rectal ACF (14). In agreement with these findings, we observed BRAF mutations in only 2% of our ACF samples. In addition, although MSI has been detected in ACF from patients with Lynch syndrome (43), sporadic ACF are known to exhibit a lower frequency of MSI (42,44,45). Accordingly, we did not observe MSI in any of the ACF samples analyzed (35).
Last, several investigations have consistently reported a high but variable incidence of KRAS mutations in sporadic ACF (15,38). The discrepancies regarding the frequency of KRAS mutations among studies could be due to the different types and sizes of study populations. Accordingly, we detected KRAS mutations in 16.3% of ACF samples; however, there was no association with the case group, similar to what has been observed previously (6,15). Moreover, MGMT promoter hypermethylation and its association with KRAS mutations have been described in the early stages of CRC (46,47). We also observed an association between KRAS mutation and MGMT promoter hypermethylation in our ACF samples, although those ACF were not predictive of CRC risk.
Before reaching definitive conclusions, some limitations should be acknowledged. First, the cross-sectional design of this study precludes us from raising any associations of causality. Second, the study cohort was composed of more males, of older age, both variables associated with CRC risk; however, we introduced them in the multivariate analysis. Another potential limitation is the presence in the control group of subjects with gastrointestinal symptoms (see Table S1, Supplementary Digital Content 1, http://links.lww.com/CTG/A46), which could be a hallmark of colonic lesions, despite a complete normal recent colonoscopy. This possibility is remote because those patients underwent a high-quality colonoscopy just before inclusion. Last, the frozen and crush artifact, orientation of the sample and the small size of the biopsies may result on scarce or no representation of the lesion, even to little alteration of the morphology and thus to the difficult pathological interpretation or limitation of an accurate histological diagnosis of some of the early lesions. In addition, by examining only the rectum, we may have limited the type of ACF samples obtained and affected our rates of prevalence. Nevertheless, in previous studies, the vast majority of these lesions are detected in the distal colon, and rectal ACF have been described as representative of the rest of the colon (10). In fact, the evaluation of proximal ACF remains limited and has resulted in the detection of few gene mutations other than KRAS or BRAF, such as EGFR orFLT3 (1,41). In addition, if a firm relationship between rectal ACF features and CRC risk would have been proven, we could use rectoscopy as a tool for identifying those patients at risk of CRC that would clearly benefit from screening with a complete colonoscopy. In particular, subjects with no rectal ACF would not need any more tests because they would be at very low risk, whereas those with dysplastic rectal ACF would need a complete colonoscopy to rule out colonic lesions. Last, by performing microdissection of our samples, we could have been able to slightly increase the frequency of the genetic alterations we observed; however, other studies have not used this technique (11,21,31,42), and the large number of samples limited the feasibility of accomplishing this with our resources. Furthermore, we considered that the methodology of our molecular analysis was sensitive enough for our purpose.
In conclusion, this large-scale study of ACF demonstrated that there is no consistent morphological characteristic that would enable us to recommend ACF as biomarkers for CRC risk. Our molecular analysis found that KRAS mutations and MGMT promoter hypermethylation might be responsible for ACF formation. Nevertheless, as none of the genomic alterations observed in ACF correlated with the CRC risk group, our results indicate that ACF might merely be preneoplastic lesions, but not suitable as an intermediate end point for CRC carcinogenesis.
CONFLICTS OF INTEREST
Guarantor of the article: Maria Pellisé, MD, PhD.
Specific author contributions: I. Quintanilla and M. López-Cerón contributed equally to this work. Study concept and design: M.P. Acquisition of clinical data: M.L.C., C.R.M., M.Z., L.M., and J.L. Pathological analysis: M.C. and M.J. Molecular analysis: I.Q., J.M., and V.A. Statistical analysis and interpretation of the data: M.L.C., I.Q., M.J., and M.P. Drafting of the manuscript: I.Q., M.C., M.J., M.L.C., and M.P. Critical revision of the manuscript for important intellectual content: J.C., F.B., A.C., M.C., and M.P.
Funding Support: This work was supported by a grant from Fondo de Investigaciones Sanitarias (FIS) del Instituto de Salud Carlos III (FIS Proyectos de Evaluación de Tecnologías Sanitarias PS09/00669). Maria López-Cerón was a research fellow from the FIS grant (Rio Hortega contract). Isabel Quintanilla is funded by the Ministerio de Educación, Cultura y Deporte (FPU12/01351). Francesc Balaguer is funded by Instituto de Salud Carlos III (PI13/00719). Maria Pellisé is funded by Instituto de Salud Carlos III (PI12/01481), Fundación Científica Asociación Española Contra el Cáncer (GCB13131592CAST), and Agència de Gestió d’Ajuts Universitaris i de Recerca (2014SGR135). Jordi Camps was awarded with a grant from the Asociación Española Contra el Cáncer, “Cofinanciado por el Fondo Europeo de Desarrollo Regional (FEDER), Unión Europea, Una manera de hacer Europa”. Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD) is funded by Instituto de Salud Carlos III. Work is supported by the Banc de Tumors-Biobanc Hospital Clinic-IDIBAPS and Xarxa de Bancs de Tumors de Catalunya (XBTC).
Potential competing interests: Maria Pellisé is a consultant for Norgine Iberia. The other authors do not have any conflict of interest.
Study Highlights.
WHAT IS KNOWN
✓ ACF have been hypothesized to be the potential precursors of colonic neoplastic lesions and have been proposed as early biomarkers for colonic carcinogenesis.
✓ They can be identified by high-definition or magnification CE as clusters of colonic crypts with a thicker epithelial lining and a dilated crypt lumen.
✓ There are many inconsistencies in the data regarding their molecular pathogenesis and their endoscopic and histological identification.
WHAT IS NEW HERE
✓ Endoscopic and molecular features were investigated in a large cohort of rectal ACF in patients at different risk of CRC.
✓ No ACF endoscopic characteristic was consistently associated with CRC risk.
✓ The molecular analysis found that KRAS mutations and MGMT promoter hypermethylation might be responsible for ACF formation. Nevertheless, none of the genomic alterations observed in ACF correlated with the CRC risk group.
TRANSLATIONAL IMPACT
✓ ACF might merely be preneoplastic lesions, but not suitable as an intermediate end point for CRC carcinogenesis.
Supplementary Material
REFERENCES
- 1.Drew DA, Devers TJ, O'Brien MJ, et al. HD chromoendoscopy coupled with DNA mass spectrometry profiling identifies somatic mutations in microdissected human proximal aberrant crypt foci. Mol Cancer Res 2014;12(6):823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: Preliminary findings. Cancer Lett 1987;37(2):147–51. [DOI] [PubMed] [Google Scholar]
- 3.McLellan EA, Bird RP. Specificity study to evaluate induction of aberrant crypts in murine colons. Cancer Res 1988;48(21):6183–6. [PubMed] [Google Scholar]
- 4.McLellan EA, Medline A, Bird RP. Dose response and proliferative characteristics of aberrant crypt foci: Putative preneoplastic lesions in rat colon. Carcinogenesis 1991;12(11):2093–8. [DOI] [PubMed] [Google Scholar]
- 5.Pretlow TP, Barrow BJ, Ashton WS, et al. Aberrant crypts: Putative preneoplastic foci in human colonic mucosa. Cancer Res 1991;51(5):1564–7. [PubMed] [Google Scholar]
- 6.Takayama T, Katsuki S, Takahashi Y, et al. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N Engl J Med 1998;339(18):1277–84. [DOI] [PubMed] [Google Scholar]
- 7.Adler DG, Gostout CJ, Sorbi D, et al. Endoscopic identification and quantification of aberrant crypt foci in the human colon. Gastrointest Endosc 2002;56(5):a128540. [DOI] [PubMed] [Google Scholar]
- 8.Roncucci L, Modica S, Pedroni M, et al. Aberrant crypt foci in patients with colorectal cancer. Br J Cancer 1998;77(12):2343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens RG, Swede H, Rosenberg DW. Epidemiology of colonic aberrant crypt foci: Review and analysis of existing studies. Cancer Lett 2007;252(2):171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho NL, Redston M, Zauber AG, et al. Aberrant crypt foci in the adenoma prevention with celecoxib trial. Cancer Prev Res (Phila) 2008;1(1):21–31. [DOI] [PubMed] [Google Scholar]
- 11.Mutch MG, Schoen RE, Fleshman JW, et al. A multicenter study of prevalence and risk factors for aberrant crypt foci. Clin Gastroenterol Hepatol 2009;7(5):568–74. [DOI] [PubMed] [Google Scholar]
- 12.Rudolph RE, Dominitz JA, Lampe JW, et al. Risk factors for colorectal cancer in relation to number and size of aberrant crypt foci in humans. Cancer Epidemiol Biomarkers Prev 2005;14(3):605–8. [DOI] [PubMed] [Google Scholar]
- 13.Bird RP, Good CK. The significance of aberrant crypt foci in understanding the pathogenesis of colon cancer. Toxicol Lett 2000;112-113:395–402. [DOI] [PubMed] [Google Scholar]
- 14.Beach R, Chan AO, Wu TT, et al. BRAF mutations in aberrant crypt foci and hyperplastic polyposis. Am J Pathol 2005;166(4):1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takayama T, Ohi M, Hayashi T, et al. Analysis of K-ras, APC, and beta-catenin in aberrant crypt foci in sporadic adenoma, cancer, and familial adenomatous polyposis. Gastroenterology 2001;121(3):599–611. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton SR, Aaltonen LA.World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. IARC Press, Lyon, France, 2000. [Google Scholar]
- 17.Pinsky PF, Fleshman J, Mutch M, et al. One year recurrence of aberrant crypt foci. Cancer Prev Res (Phila) 2010;3(7):839–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson JC, Swede H, Rustagi T, et al. Aberrant crypt foci as predictors of colorectal neoplasia on repeat colonoscopy. Cancer Causes Control 2012;23(2):355–61. [DOI] [PubMed] [Google Scholar]
- 19.Hurlstone DP, Karajeh M, Sanders DS, et al. Rectal aberrant crypt foci identified using high-magnification-chromoscopic colonoscopy: Biomarkers for flat and depressed neoplasia. Am J Gastroenterol 2005;100(6):1283–9. [DOI] [PubMed] [Google Scholar]
- 20.Roncucci L, Stamp D, Medline A, et al. Identification and quantification of aberrant crypt foci and microadenomas in the human colon. Hum Pathol 1991;22(3):287–94. [DOI] [PubMed] [Google Scholar]
- 21.Cohen G, Mustafi R, Chumsangsri A, et al. Epidermal growth factor receptor signaling is up-regulated in human colonic aberrant crypt foci. Cancer Res 2006;66(11):5656–64. [DOI] [PubMed] [Google Scholar]
- 22.Pretlow TP, Pretlow TG. Mutant KRAS in aberrant crypt foci (ACF): Initiation of colorectal cancer? Biochim Biophys Acta - Rev Cancer 2005;1756(2):83–96. [DOI] [PubMed] [Google Scholar]
- 23.Suehiro Y, Hinoda Y. Genetic and epigenetic changes in aberrant crypt foci and serrated polyps. Cancer Sci 2008;99(6):1071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seike K, Koda K, Oda K, et al. Assessment of rectal aberrant crypt foci by standard chromoscopy and its predictive value for colonic advanced neoplasms. Am J Gastroenterol 2006;101(6):1362–9. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Ceron M, Pellise M. Review article: Biology and diagnosis of aberrant crypt foci. Color Dis Off J Assoc Coloproctology Gt Britain Irel. 2011. [DOI] [PubMed] [Google Scholar]
- 26.Alrawi SJ, Carroll RE, Hill HC, et al. Genomic instability of human aberrant crypt foci measured by inter-(simple sequence repeat) PCR and array-CGH. Mutat Res Mol Mech Mutagen 2006;601(1–2):30–8. [DOI] [PubMed] [Google Scholar]
- 27.Schoen RE, Mutch M, Rall C, et al. The natural history of aberrant crypt foci. Gastrointest Endosc 2008;67(7):1097–102. [DOI] [PubMed] [Google Scholar]
- 28.Moreira L, Muñoz J, Cuatrecasas M, et al. Prevalence of somatic mutl homolog 1 promoter hypermethylation in Lynch syndrome colorectal cancer. Cancer 2015;121(9):1395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008;26(35):5705–12. [DOI] [PubMed] [Google Scholar]
- 30.Patai Á V, Molnár B, Tulassay Z, et al. Serrated pathway: Alternative route to colorectal cancer. World J Gastroenterol 2013;19(5):607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta AK, Pinsky P, Rall C, et al. Reliability and accuracy of the endoscopic appearance in the identification of aberrant crypt foci. Gastrointest Endosc 2009;70(2):322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kowalczyk M, Siermontowski P, Mucha D, et al. Chromoendoscopy with a standard-resolution colonoscope for evaluation of rectal aberrant crypt foci. Wang H, editor. PLoS One. 2016;11(2):e0148286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouzourene H, Chaubert P, Seelentag W, et al. Aberrant crypt foci in patients with neoplastic and nonneoplastic colonic disease. Hum Pathol 1999;30(1):66–71. [DOI] [PubMed] [Google Scholar]
- 34.Kristt D, Winston GJ, Mellov MM, et al. Patterns of proliferative changes in crypts bordering colonic tumors: Zonal histology and cell cycle marker expression. Pathol Oncol Res 1999;5(4):297–303. [DOI] [PubMed] [Google Scholar]
- 35.Shpitz B, Bomstein Y, Mekori Y, et al. Aberrant crypt foci in human colons: Distribution and histomorphologic characteristics. Hum Pathol 1998;29(5):469–75. [DOI] [PubMed] [Google Scholar]
- 36.Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology 2005;47(1):32–40. [DOI] [PubMed] [Google Scholar]
- 37.Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: A prospective study of patients undergoing colonoscopy. Gastroenterology 2006;131(5):1400–7. [DOI] [PubMed] [Google Scholar]
- 38.Smith AJ, Stern HS, Penner M, et al. Somatic APC and K-ras codon 12 mutations in aberrant crypt foci from human colons. Cancer Res 1994;54(21):5527–30. [PubMed] [Google Scholar]
- 39.Otori K, Konishi M, Sugiyama K, et al. Infrequent somatic mutation of the adenomatous polyposis coli gene in aberrant crypt foci of human colon tissue. Cancer 1998;83(5):896–900. [DOI] [PubMed] [Google Scholar]
- 40.Inoue A, Okamoto K, Fujino Y, et al. B-RAF mutation and accumulated gene methylation in aberrant crypt foci (ACF), sessile serrated adenoma/polyp (SSA/P) and cancer in SSA/P. Br J Cancer 2015;112(2):403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mo A, Jackson S, Varma K, et al. Distinct transcriptional changes and epithelial-stromal interactions are altered in early-stage colon cancer development. Mol Cancer Res 2016;14(9):795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenberg DW, Yang S, Pleau DC, et al. Mutations in BRAF and KRAS differentially distinguish serrated versus non-serrated hyperplastic aberrant crypt foci in humans. Cancer Res 2007;67(8):3551–4. [DOI] [PubMed] [Google Scholar]
- 43.Staffa L, Echterdiek F, Nelius N, et al. Mismatch repair-deficient crypt foci in Lynch syndrome: Molecular alterations and association with clinical parameters. PLoS One 2015;10(3):e0121980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan AO, Broaddus RR, Houlihan PS, et al. CpG island methylation in aberrant crypt foci of the colorectum. Am J Pathol 2002;160(5):1823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heinen CD, Shivapurkar N, Tang Z, et al. Microsatellite instability in aberrant crypt foci from human colons. Cancer Res 1996;56(23):5339–41. [PubMed] [Google Scholar]
- 46.Hibi K, Goto T, Mizukami H, et al. MGMT gene is aberrantly methylated from the early stages of colorectal cancers. Hepatogastroenterology 2009;56(96):1642–4. [PubMed] [Google Scholar]
- 47.Menigatti M, Pedroni M, Verrone AM, et al. O6-methylguanine-DNA methyltransferase promoter hypermethylation in colorectal carcinogenesis. Oncol Rep 2007;17(6):1421–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.