Figure 4.
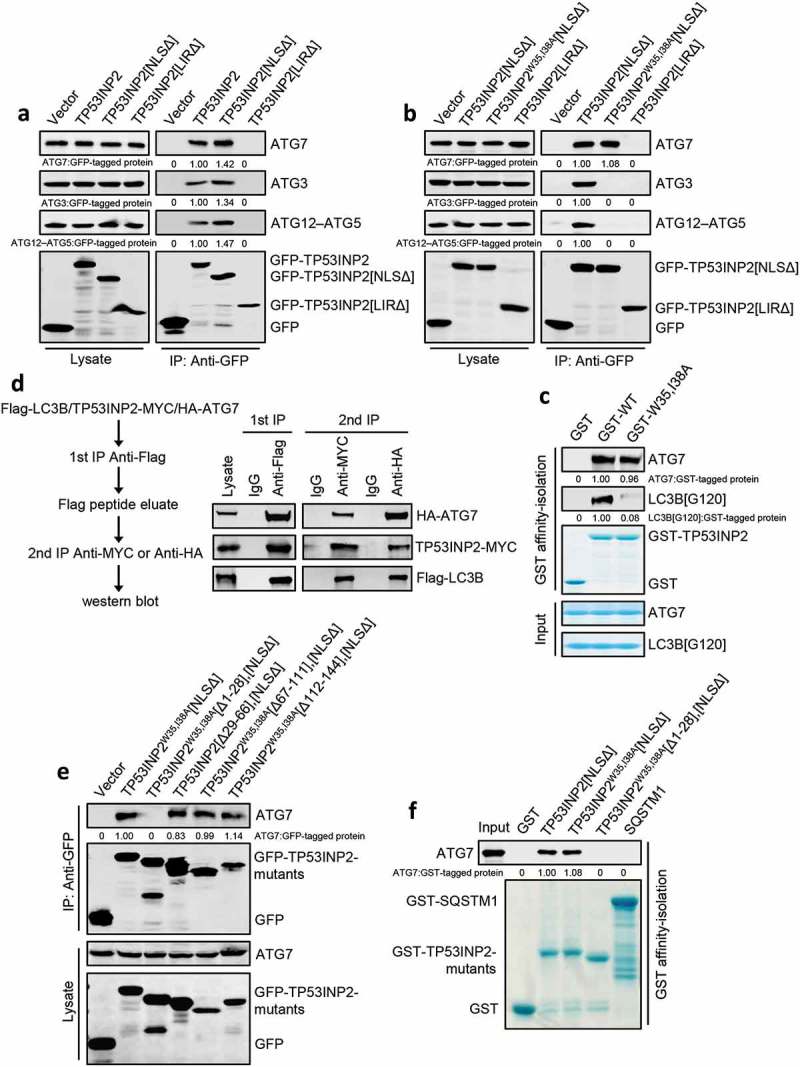
TP53INP2 forms a complex with LC3B and ATG7. (a) Coimmunoprecipitation of ATG7, ATG3 or ATG12–ATG5 with GFP-TP53INP2, GFP-TP53INP2[NLSΔ] or GFP-TP53INP2[LIRΔ] from HEK293 cells. TP53INP2 proteins were immunoprecipitated by anti-GFP. The coprecipitated ATG7, ATG3 or ATG12–ATG5 was detected by western blot using anti-ATG3, anti-ATG7 or anti-ATG5 respectively. (b) Coimmunoprecipitation of ATG7, ATG3 or ATG12–ATG5 with GFP-tagged TP53INP2[NLSΔ], TP53INP2W35,I38A[NLSΔ] or TP53INP2[LIRΔ]. GFP-tagged TP53INP2 mutants were immunoprecipitated using anti-GFP and the precipitates were analyzed using anti-ATG7, anti-ATG3 or anti-ATG5. (c) In vitro TP53INP2-ATG7 binding assay. Purified GST-TP53INP2 or GST-TP53INP2W35,I38A was incubated with purified LC3B[G120] and ATG7. After affinity-isolating GST-TP53INP2 or GST-TP53INP2W35,I38A with glutathione-sepharose 4B beads, the bound LC3B[G120] and ATG7 were analyzed by western blot. (d) HEK293T cells were cotransfected with Flag-LC3B, TP53INP2-MYC and HA-ATG7. The cells were lysed 48 h after transfection and Flag-LC3B was immunoprecipitated with anti-Flag. After incubation of the Flag-LC3B precipitates with Flag peptide, the eluate was used for immunoprecipitation with either anti-MYC or anti-HA. The immunoprecipitates were then analyzed by western blot by anti-Flag, anti-MYC and anti-HA respectively. (e) Coimmunoprecipitation of ATG7 with each of the indicated GFP-tagged truncated TP53INP2 mutants in HEK293 cells. TP53INP2 proteins were immunoprecipitated using anti-GFP and the precipitates were analyzed using anti-ATG7. (f) Purified GST-tagged TP53INP2[NLSΔ], TP53INP2W35,I38A[NLSΔ], TP53INP2W35,I38A[Δ1-28],[NLSΔ] or SQSTM1 was incubated with purified ATG7, then the GST-tagged proteins were affinity-isolated by glutathione-sepharose 4B beads and bound ATG7 was detected by western blot using anti-ATG7.
