Fig. 5.
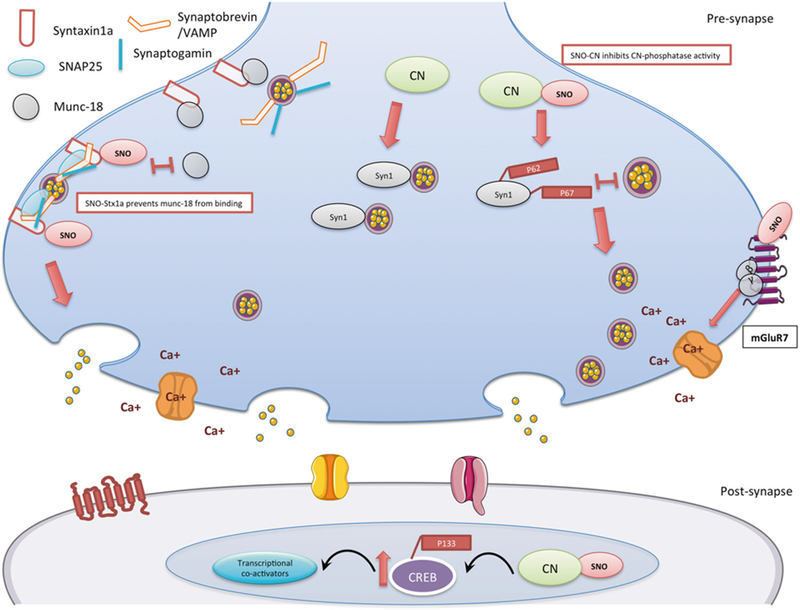
Schematic model summarizing the findings of the SNO-proteomics analysis and its consequences in 4m-cor-KO mice. In the pre-synapse, right side: S-nitrosylation of mGluR7 inhibits the receptor activity, once mGluR7 in a rest (or inhibited), β and γ subunits will not move to the calcium channel and inhibit the calcium channel activity. SNO-mGluR7 may lead to increase of calcium influx in the pre-synapse. In the pre-synapse, middle: S-nitrosylation of calcineurin (CN) leads to the inhibition of the phosphatase activity. This leads to an increase of its phosphorylated substrate P-synapsin1 (Syn1) (Ser62, Ser67). Phosphorylation of synapsin1 disconnects it from the vesicle and this leads to vesicle mobilization from the reserved pool to the readily released pool. In the pre-synapse, left side: SNO-Syntaxin1a prevents munc18 (also known as n-sec1) from binding to the closed conformation of syntaxin1a once SNOed. This allows syntaxin1a to unfold and bind to both VAMP on the vesicle and SNAP25 at the release site, which in turn enables the vesicle to dock to the membrane. In the post-synapse: The prolonged phosphorylation of CREB on Ser133 is a process mediated by Ca2+ dependent and -independent protein kinases. CREB phosphorylation is terminated by CN possibly through direct CREB dephosphorylation. SNO-CN inhibits CN activity and leads to increase of P-CREB (Ser 133) that leads to an increase of recruiting transcriptional co-activators
