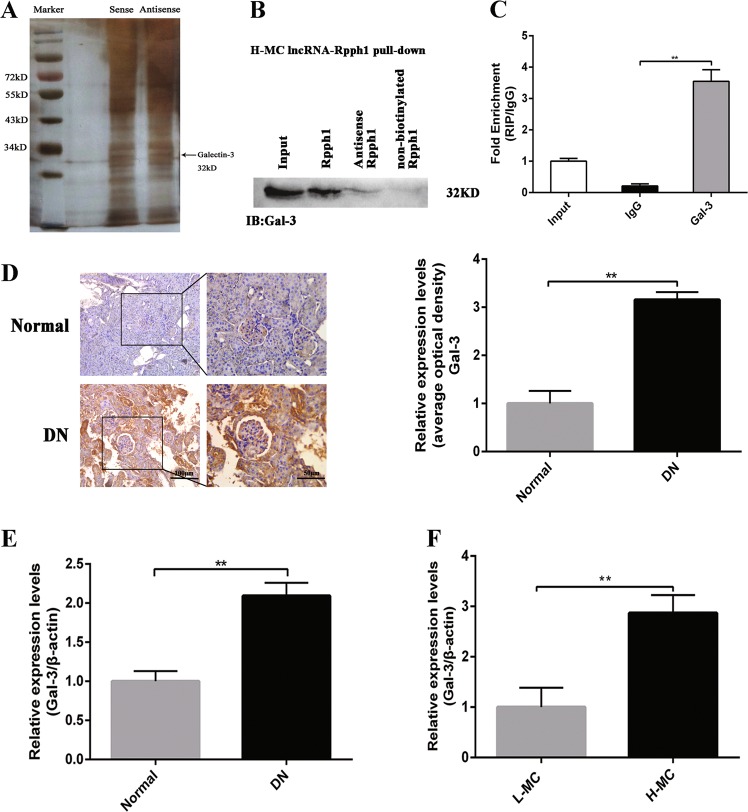
Fig. 3. Rpph1 (ribonuclease P RNA component H1) interacted with Gal-3.
a RNA pull-down assay was performed to detect the proteins interacting with Rpph1; mesangial cell (MC) lysates cultured under high glucose conditions were incubated with 5′ end biotin-labeled sense, antisense lncRNA Rpph1 (negative control), or non-biotin-labeled lncRNA Rpph1, and mass spectrometry and silver staining were performed to identify the interacting proteins. Black arrow: the Gal-3 band. b Gal-3 was detected in biotin-labeled sense, antisense lncRNA Rpph1, and non-biotin-labeled lncRNA Rpph1 groups by Western blot after pull-down; the data are representative of three independent experiments. Data are presented as mean ± SD. **P < 0.01. c The enrichment ratio of Rpph1 with Gal-3 antibody compared with immunoglobulin G (IgG), as determined by RNA immunoprecipitation (RIP) assay; the data are representative of three independent experiments. Data are presented as mean ± SD. **P < 0.01. d The protein levels of Gal-3 in renal tissues of diabetic nephropathy (DN) mice and normal controls was detected by immunohistochemistry and quantitative analysis; the data are representative of three independent experiments. Data are presented as mean ± SD. **P < 0.01. e The expression levels of Gal-3 in the renal tissue of DN mice and normal controls were detected by quantitative real-time PCR (qRT-PCR); the data are representative of three independent experiments. Data are presented as mean ± SD. **P < 0.01. f The expression of Gal-3 in H-MC and L-MC was detected by qRT-PCR; the data are representative of three independent experiments. Data are presented as mean ± SD. **P < 0.01