Abstract
Background: Atherosclerosis remains a leading cause of cardiology disease worldwide, which vascular smooth muscle cells (VSMCs) proliferation and apoptosis are involved. Increasing evidences have revealed that long non-coding RNAs (lncRNAs) considered to be critical regulatory factors of VSMCs function. However, the molecular mechanism is not fully understood. Methods: First, we establish the ox-LDL induced VSMC model. We conducted RT-PCR to measure MEG3 expression and miR-361-5p expression in this model. The proliferation and apoptosis of VSMCs were measured via CCK-8 proliferative assay and flow cytometry respectively. We used knockdown and overexpression system to identify the molecular mechanism. In addition, luciferase report assay and bioinformatics analysis were used to confirm the bio-target of different factors. Results: LncRNA MEG3 was down-regulated and related with miR-361-5p expression in ox-LDL injured VSMCs. Inhibition of lncRNA MEG3 promotes the proliferation and decelerates apoptosis of VSMCs. Moreover, MEG3 acts as a competing endogenous RNA (ceRNA) for miR-361-5p and further regulate ABCA1 expression regulate proliferation and apoptosis in ox-LDL injured VSMCs. Conclusion: These results suggest that LncRNA MEG3 regulate proliferation and apoptosis in ox-LDL injured VSMCs and function as a ceRNA for miR-361-5p to modulate ABCA1 expression.
Keywords: LncRNA MEG3, proliferation, apoptosis, ABCA1, vascular smooth muscle cells
Introduction
Despite the remarkable therapeutic progress archived in cardiology, atherosclerosis remains a main cause of stroke and cardiovascular death [1,2]. Atherosclerosis is known as a complex pathophysiological process which involved multiple cellular and molecular changes [3]. It is well-known that vascular smooth muscle cells (VSMCs) play an essential role in atherosclerosis [4,5]. For instance, the majority of VSMCs in the plaque are derived from the medial layer of the vessel which act as a regulator on atherosclerotic plaque, also, proliferation of VSMCs promoting atherogenesis as a response to injury [6,7]. Additionally, VSMCs apoptosis occurs in physiological vessel remodeling, which may induce vulnerability of plaque [8-10]. Thus, understanding the deep molecular mechanisms involved in those pathophysiological processes in VSMCs may provide a novel concept for treating atherosclerosis-related disease.
Long non-coding RNAs (lncRNAs) constitute a cluster of transcripts longer than 200 nucleotides without protein-coding ability, however, they are able to regulate gene expression at transcription, epigenetic, and translation levels [11-13]. Increasing evidence suggest that lncRNAs are involved in multiple cellular signaling pathway including cell differentiation [14], proliferation [15], migration [16] and apoptosis [17]. Recent research have shown that lncRNAs are highly expressed in VSMCs, and these lncRNAs have been demonstrated serves in different biological pathways. For instance, lncRNA RNCR3 accelerates the apoptosis in atherosclerosis process, meanwhile, it also inhibits the proliferation and migration by regulating Kruppel-like factor 2 and miR-185-5p [18]. In addition, lncRNA GAS5 regulate hypertension-induced vascular remodeling through β-catenin signaling pathway [19]. Zhao et al focus on another lncRNA named MYOSLID, they confirmed that MYOSLID is a novel modulator in VSMC differentiation program via regulating transforming growth factor-β/SMAD pathways [20].
One of the most novel functions of lncRNAs is that they are able to serve as ceRNA which competes with coding gene RNA to be targeted with microRNA (miRNA), thus regulate the function of these genes [21,22]. So far, in many studies, the ability of lncRNAs to associate with miRNAs has been partly investigated. Using RNA-sequencing, Lnc-Ang362 was identified in VSMCs, Lnc-Ang362 functions as the host transcript for miR-221 and miR-222, which is responsible for VSMCs proliferation [23]. In addition, another study demonstrated that LncRNA UCA1 sponges miR-26a and further modulate VSMCs migration and proliferation [24]. LncRNA expressed gene 3 (MEG3) has been proven regulates VSMC migration and apoptosis, thus plays a function role in vascular transformation. However, there is lack of the evidence of LncRNA MEG3 and miRNA interaction mechanism.
In this study, we would like to explore the role of lncRNA MEG3 in ox-LDL injured VSMCs model. The functions of MEG3 and its down-stream factors were determined by functional experiments. Our study may provide a new perspective on atherosclerosis treatment.
Methods and materials
Cell culture
The VSMCs were cultured in DMEM with supplemented 10% FBS and 1% antibiotics (penicillin and streptomycin). Meanwhile, 10 ng/ml of fibroblast growth factor was added in the culture medium. The temperature of container was maintained at 37°C with 5% CO2 in the humidified environment. Culture medium was changed every 3 days. VSMCs from passages 3 were used for in vitro experiments. Ox-LDL was used for treatment of VSMCs, different concentrations of ox-LDL were added into DMEM 24 h before measurement. For human embryonic kidney 293T cells, the culture medium and condition were the same as VSMCs as previously described. 293T cells were used for transfection.
qRT-PCR
The total RNA was extracted from treated VSMCs by using RNA isolation kit (Invitrogen, USA) according to the manufacturer’s instructions. Next, RNA was transcribed into complementary DNA (cDNA). The primers in our study were described in Table 1. The cDNA synthesized was used to perform PCR on ABI 7500 fast Real-Time PCR System. U6 and GAPDH were used as control. Relative levels of gene expression were calculated via 2-ΔΔCt method.
Table 1.
Primers used in this study
| Gene | Primer sequence |
|---|---|
| miR-361-5p | Forward: 5’-ATAAAGTGCTGACAGTGCAGATAGTG-3’ |
| Reverse: 5’-TCAAGTACCCACAGTGCGGT-3’ | |
| U6 | Forward: 5’-CTCGCTTCGGCAGCACA-3’ |
| Reverse: 5’-AACGCTTCACGAATTTGCGT-3’ | |
| MEG3 | Forward: 5’-CTGCCCATCTACACCTCACG-3’ |
| Reverse: 5’-CTCTCCGCCGTCTGCGCTAGGGGCT-3’ | |
| PCNA | Forward: 5’-CGACTGCTTAAGATTTCGAGGCGCGCCTGGTCCAG-3’ |
| Reverse: 5’-CCTATCGCTAGCTCCAGCTCCACCCGCAGATCCTTCTTCATCC-3’ | |
| GAPDH | Forward: 5’-AGTCCACTG GCGTCTTCA-3’ |
| Reverse: 5’-GAGTC CTTCCACGATACCAA-3’ | |
| α-SMA | Forward: 5’-CCACCGCAAATGCTTCTAAGT-3’ |
| Reverse: 5’-GGCAGGAATGATTTGGAAAGG-3’ | |
| SM22-α | Forward: 5’-GCUAGUGGAGUGGAUUGUATT-3’ |
| Reverse: 5’-UACAAUCCACUCCACUAGCTT-3’ |
Cell transfection
For transfection, The VSMCs were seeded into 24-well plates, and incubated with DMEM supplemented with 10% FBS only. Then we transfected pcDNA-MEG3 vector, si-lncRNA MEG3, miR-361-5p mimic, miR-361-5p inhibitor using Lipofectamine 2000 reagent (Invitrogen, USA) for 48 h based on manufacturer’s protocols. After transfection, total RNA and protein were extracted from VSMCs for further use.
Dual luciferase reporter assay
For luciferase reporter assays, the 3’-UTR of MEG3 containing miR-361-5p binding sites were termed as pGLO-MEG3-WT/Mut luciferase vector. 24 h later, we transfected these factors into HEK-293T cells. 36 h after the transfection, the cells were lysed and tested using luciferase system. Dual luciferase reporter system was used based on manufacturer’s protocols.
Annexin V detection of apoptosis
Following transfection, the VSMCs were harvested by trysin without EDTA. After washing with PBS, these cells were resuspended in 1 × Annexin V binding buffer at a concentration of 1 × 106 cells/ml. Then we added 5 ml FITC-Annexin V to each tube and used propidium iodide (PI) at darkness for 15 min. Finally, we conducted flow cytometry (Becton-Dickinson, USA) with stained VSMCs.
Cell proliferation assay
The cell proliferation was measured by CCK-8 assay. Different groups of VSMCs were cultured in 96-well plates. After 48 h of culturing, CCK-8 reagent (10 μl) was added into each well and incubated according to the manufacturer’s instructions. By using micro-plate reader, absorbance was measured at wavelength of 450 nm 72 h after treatment. All tests were repeated at least 3 times.
Western blot
The VSMCs were washed with cold PBS, then, these cells were lysed in RIPA. Next, total protein samples were extracted. All samples from different groups were used for western blotting. The protein samples were incubated with the primary antibody at 4°C overnight include ABCA1, PCNA, α-SMA, SM22-α, GAPDH (1:1000 dilution, Sigma, USA). After that, samples were incubated with secondary antibodies (1:1000 dilution, Sigma, USA). All data were analyzed and quantified with Image-J software.
RNA immunoprecipitation (RIP)
For RNA immunoprecipitation, the different groups of cells were collected and lysed into RIP buffer. Later, cell lysates were incubated with RIP buffer containing magnetic beads conjugated with human anti-Ago2 antibody or negative control IgG (anti-IgG). After immune-precipitated RNA was extracted, the RT-PCR was conducted to measure the purified RNA including expression of MEG3 and miR-361-5p.
Statistical analysis
All data were imported into SPSS 21.0 software (IBM, USA). Results were presented as mean ± SEM. Comparisons between groups were made by Mann-Whitney U test or Student’s t test. Comparisons among more than two groups were using one-way ANOVA. P value less than 0.05 was considered statistically significant.
Results
LncRNA MEG3 and miR-361-5p expression are correlated in ox-LDL injured VSMCs
First of all, we would like to confirm the effect of ox-LDL in VSMCs. VSMCs were incubated with ox-LDL in different dosage (25-200 μg/ml) at different time point (12-72 h). The results were shown in Figure 1A and 1B, we found that at 75 μg/ml and 100 μg/ml in 48 h, the proliferation of VSMCs was significantly increased. The results suggested that ox-LDL induced VSMCs proliferation showed both time- and dose-dependent manner. Based on these data, we decided to use 100 μg/ml ox-LDL treatments at 48 h for the following experiments. Subsequently, in order to address the expression pattern of lncRNA MEG3 and miR-361-5p, we used RT-PCR to detect gene expression in ox-LDL induced VSMCs model. As shown in Figure 1C, the expression of MEG3 was significantly decreased in ox-LDL injured VSMCs. Furthermore, we assessed the miR-361-5p expression, we found that miR-361-5p was up-regulated as shown in Figure 1D. We conducted Pearson’s correlation to address the correlation between lncRNA MEG3 and miR-361-5p, the data suggested that the expression of lncRNA MEG3 and miR-361-5p have negative correlation (Figure 1E). These data demonstrated that MEG3 is down-regulated and related with miR-361-5p expression in ox-LDL treated VSMCs.
Figure 1.
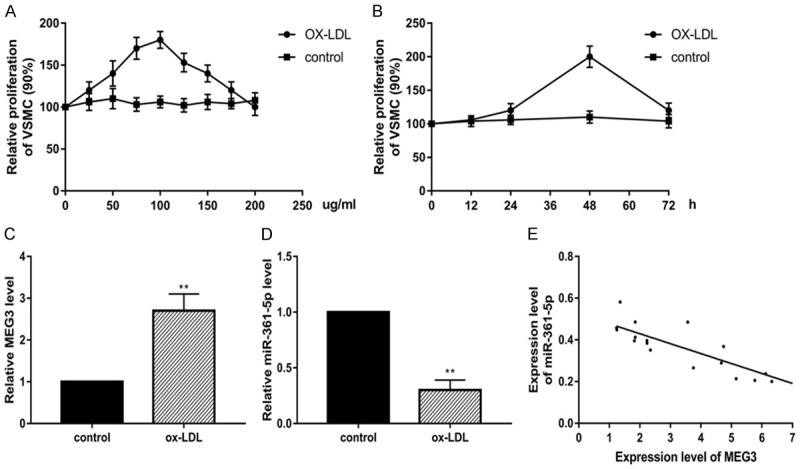
LncRNA MEG3 down-regulation is related with miR-361-5p expression in ox-LDL induced VSMCs. (A) ox-LDL induce VSMCs proliferation at time-dependent ox-LDL (25-100 μg/ml) (B) ox-LDL induce VSMCs proliferation at dose-dependent manner (6-72 h). Real-time PCR assay for the expression of MEG3 (C) and miR-361-5p (D). The correlation between MEG3 and miR-361-5p was shown in (E). Data were represented as mean ± SEM. *P<0.05, **P<0.01, and ***P<0.001.
Inhibition of lncRNA MEG3 promotes the proliferation and decelerates apoptosis in VSMCs
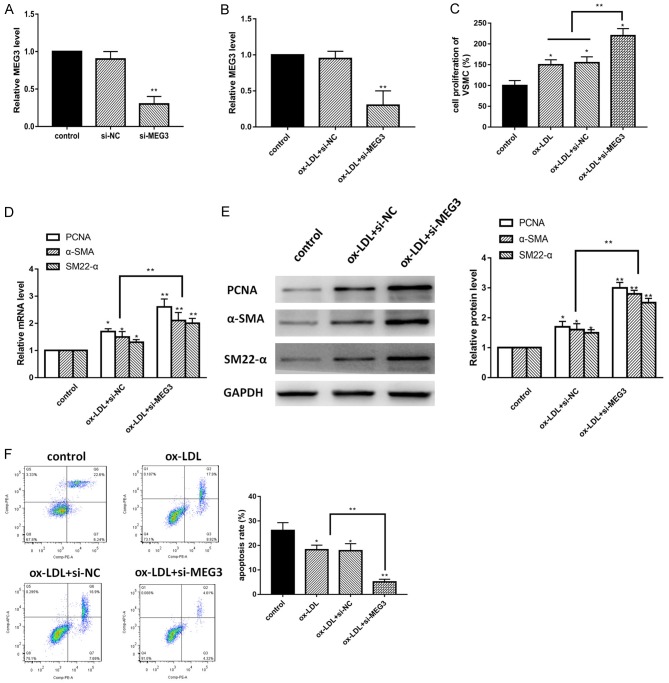
Previously, we confirmed that lncRNA MEG3 was down-regulated in ox-LDL treated VSMCs, in order to identify the specific role of lncRNA MEG3 in ox-LDL induced model, we conducted a series of loss-of-function experiments by using si-MEG3. First, we detected the expression of lncRNA MEG3 after transfection by using RT-PCR, the expression of lncRNA MEG3 was suppressed in si-MEG3 group in both normal and ox-LDL conditions compared with control group (Figure 2A and 2B). Then, we used CCK-8 assay to measure the proliferation of VSMCs. Our data identified that knockdown of MEG3 drastically promoted the proliferation of ox-LDL treated VSMCs (Figure 2C). To further confirm the hypothesis, we conducted western blot and RT-PCR to measure the biomarkers of proliferation such as proliferating cell nuclear antigen (PCNA), α-smooth muscle actin (α-SMA), smooth muscle 22α (SM22-α). The results were shown in Figure 2D and 2E, in both protein and mRNA level, PCNA and α-SMA expression were remarkably increased in si-MEG3 group, which suggest that knockdown of MEG3 promoted the proliferative capability of ox-LDL induced VSMCs. In addition, flow cytometry was used for detecting cell apoptosis. As shown in Figure 2F, compared with control group, knockdown of MEG3 decreased the apoptosis process. Taken together, these data clarified that inhibition of lncRNA MEG3 promoted the proliferation and inhibited apoptosis of ox-LDL treated VSMCs.
Figure 2.
Inhibition of lncRNA MEG3 decelerates the proliferation and apoptosis of VSMCs. A. Real-time PCR showed the expression of MEG3 in VSMCs in normal condition. B. Real-time PCR showed the expression of MEG3 in VSMCs under ox-LDL treatment. C. Cell proliferation was detected by CCK-8 assay in different groups. D. PCNA, α-SMA and SM22-α mRNA were measured by RT-PCR. E. Western blot assay was used for measurement of proliferation biomarkers including PCNA, α-SMA and SM22-α. F. Flow cytometry was used to assay the apoptosis of VSMCs. Data were represented as mean ± SEM. *P<0.05, **P<0.01, and ***P<0.001.
miR-361-5p targeted 3’-UTR of LncRNA MEG3
Our results showed lncRNA MEG3 and miR-361-5p have negative correlation, in order to further understand the deep molecular mechanism and address the relationship between LncRNA MEG3 and miR-361-5p. LncRNAs can serve as regulatory factor through targeting miRNAs. A bioinformatics analysis revealed that lncRNA MEG3 contains one conserved target site of miR-361-5p as shown in Figure 3A. In order to further confirm the predicted target, we used dual-luciferase assay and RT-PCR assay, as shown in Figure 3B, the results indicated that luciferase activity was significantly decreased in pGLO-MEG3-WT group, however, there was no change in pGLO-MEG3-MUT group. In addition, we detect expression of miR-361-5p in si-MEG3 model. The results demonstrated that knockdown of MEG3 remarkably increased the expression of miR-361-5p in ox-LDL treated VSMCs model (Figure 3C). To further find out whether MEG3 and miR-361-5p were associated through miRNA ribonucleoprotein complexes, we used RNA immunoprecipitation, the data demonstrated that MEG3 and miR-361-5p were increased in the Ago2-containing miRNAs compared with the control group (Figure 3D). Overall, our results identified that miR-361-5p is a direct target of lncRNA MEG3 in ox-LDL treated VSMCs.
Figure 3.
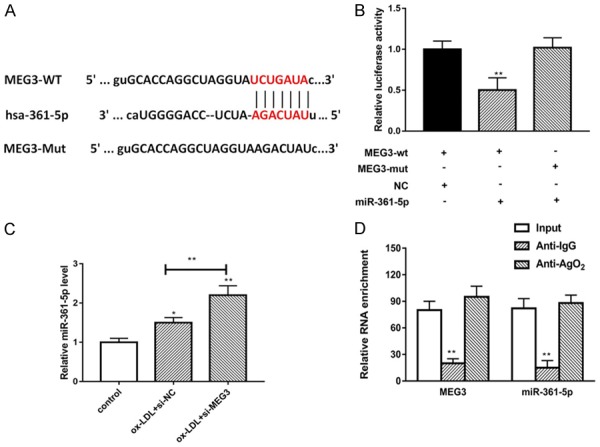
miR-361-5p targeted 3’-UTR of LncRNA MEG3. A. The putative miR-361-5p binding sequence of the wild type and mutation sequence of MEG3. B. Luciferase activity of a luciferase reporter plasmid (pluc) containing wild-type or mutant MEG3 3’UTR co-transfected with miR-361-5p was determined by the dual luciferase assay. C. The expression of miR-361-5p was detected in different groups by using RT-PCR. D. Cellular lysates from VSMC cells were used for RIP with an Ago2 antibody and IgG antibody. The levels of MEG3 and miR-361-5p were detected by qRT-PCR. Data were represented as mean ± SEM. *P<0.05, **P<0.01, and ***P<0.001.
LncRNA MEG3-derived miR-361-5p directly targets on ABCA1
Subsequently, bioinformatics reports showed that miR-361-5p shared complementary binding sites with 3’-UTR of ABCB1 mRNA, as shown in Figure 4A. In addition, ABCB1 already confirmed to be a direct target of miR-361-5p in different studies. Therefore, ABCA1 may act as a target of miR-361-5p. To this end, we co-transfected pluc-ABCA1 3’UTR-WT with miR-361-5p into HEK293T cells for a luciferase assay. The results showed that miR-361-5p targeted with 3’-UTR of ABCA1 mRNA (Figure 4B). Next, we would like to find out the relationship among ABCA1 and miR-361-5p, the cells were transfected with miR-361-5p mimic and miR-361-5p inhibitor, the results indicated that the mRNA expression of ABCA1 were up-regulated by miR-361-5p mimic and down-regulated by miR-361-5p inhibitor, as shown in Figure 4C. The protein level tested by western blot showed that, miR-361-5p mimic down-regulate ABCA1 protein expression, while miR-361-5p inhibitor promote ABCA1 protein expression, which has the opposite effect (Figure 4D). To conclude, these data indicated that LncRNA MEG3 positively regulated ABCA1 expression via regulate miR-361-5p, suggesting that lncRNA MEG3 may act as an endogenous ‘sponge’ by binding miR-361-5p, thus abolishing the miRNA-mediated repressive activity on the ABCA1 3’UTR.
Figure 4.
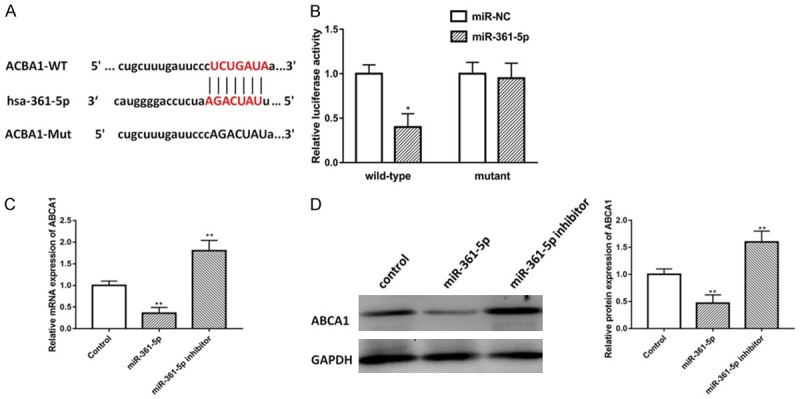
LncRNA MEG3-derived miR-361-5p directly targets on ABCA1. A. The putative ABCA1 binding sequence of the wild type and mutation sequence of miR-361-5p. B. Luciferase activity of a luciferase reporter plasmid (pluc) containing wild-type or mutant ABCA1 3’UTR co-transfected with miR-361-5p was determined by the dual luciferase assay. C. Real-time PCR was used to determine ABCA1 mRNA expression in miR-361-5p mimic and miR-361-5p inhibitor groups. D. Western blot analysis of the protein levels of miR-361-5p target genes ABCA1 in VSMCs transfected with miR-361-5p mimic and miR-361-5p inhibitor. Data were represented as mean ± SEM. *P<0.05, **P<0.01, and ***P<0.001.
Inhibition of LncRNA MEG3 promotes VSMCs proliferation and inhibits apoptosis via modulating miR-361-5p
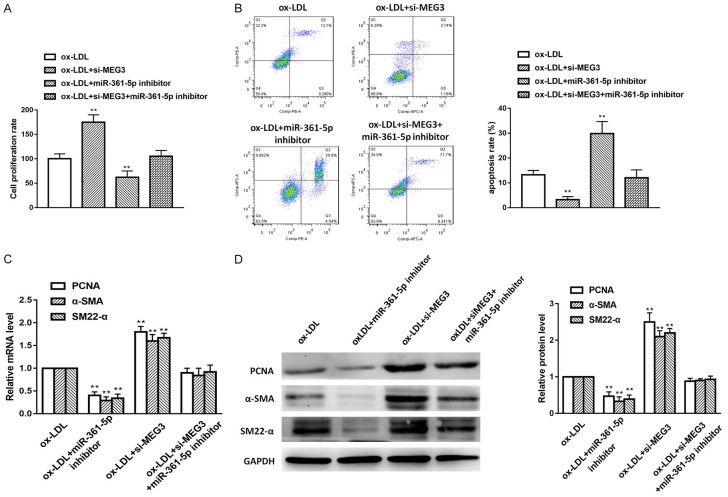
Finally, we would like to explore whether lncRNA MEG3 regulate VSMCs proliferation and apoptosis by modulating miR-361-5p, we designed the following experiments. We set up groups as ox-LDL, ox-LDL+iR-361-5p inhibitor, ox-LDL+si-MEG3 and ox-LDL+si-MEG3+miR-361-5p inhibitor, and CCK-8 cell proliferation assays, RT-PCT, western blot and flow cytometry assay were performed. As shown in Figure 5A and 5B, miR-361-5p inhibition group showed lower cell survival rate and higher apoptotic rate compared with the si-MEG3 only group. Moreover, we measured protein level and mRNA level of PCNA, α-SMA and SM22-α in different transfected groups, we found that both protein level and mRNA level of these factors were significantly increased in si-MEG3 group, However, miR-361-5p inhibitor counteract the effect of si-MEG3 (Figure 5C and 5D). To sum up, these data revealed that lncRNA MEG3 suppresses VSMCs proliferation and apoptosis via modulating miR-361-5p.
Figure 5.
LncRNA MEG3 attenuate VSMCs proliferation and apoptosis by regulating miR-361-5p. A. CCK-8 assay was used to detect the cell proliferation in different groups. B. Flow cytometry was used to detect the cell proliferation in different groups. C. CNA, α-SMA and SM22-α mRNA were measured by RT-PCR. D. Western blot assay was used for measurement of proliferation biomarkers including PCNA, α-SMA and SM22-α. Data were represented as mean ± SEM. *P<0.05, **P<0.01, and ***P<0.001.
Discussion
VSMCs proliferation and apoptosis have been considered to be important processes in multiple vascular diseases including atherosclerosis, aneurysm formation, and transplant arteriopathy [10,25]. They also play key roles in pathological vessel remodeling [26]. VSMC apoptosis and proliferation occurs within hours to days due to blood flow changes in pathological condition, it may last for the whole process [27,28]. There are many molecular factors have been confirmed contributing to VSMCs proliferation and apoptosis. However, the endogenous pathways and deep mechanism of these processes were not fully understood. In this study, we sought to confirm the potential role of MEG3 and its downstream factors and their relationship in the ox-LDL treated VSMCs model.
For the underline mechanisms of proliferation and apoptosis in VSMCs, lncRNAs or miRNAs are considered to be novel regulators in the past decade. Recently, research on the interactions between lncRNAs and miRNAs attract much attention. Among these lncRNAs and miRNAs, lncRNA MEG3 is a new factor in this field which remain unclear. MEG3 locus on human chromosome 14q32, which was originally clarified as a regulator of tumor suppressor involved in many diseases [29,30]. To date, many studies have shown that MEG3 was involved in cell proliferation and apoptosis in different pathological processes. In addition, MEG3 also confirmed to be a regulator in cell migration and invasion in other studies [31,32]. Numerous evidences identified that MEG3 suppresses cell proliferation and apoptosis mainly via p53-dependent pathways [33-35], other pathways such as Wnt signaling pathway [36], PI3k/Akt pathway [37,38], are also confirmed involved in proliferation or apoptosis. In VSMCs, Liu et al demonstrated that MEG3 is regulated by dNK-derived IFN-γ and further modulate VSMC migration and apoptosis [39]. However, until now, there is no study to investigate the role of MEG3 in lncRNA and miRNA interaction in VSMCs.
Our study demonstrated a novel lncRNA-miRNA-mRNA regulatory network. In this study, at the very beginning, we established the ox-LDL induced VSMCs model to mimic the pathological condition. We identified that the expression of lncRNA MEG3 was decreased, however, the expression of miR-361-5p showed opposite tendency. Based on these results, we found there was a negatively correlation between lncRNA MEG3 and miR-361-5p. By using bioinformatics analysis and luciferase report assay, we identified that miR-361-5p is a direct target of lncMEG3. Furthermore, a series of gain- and loss-function experiments were conducted to confirm the role of lncMEG3 in ox-LDL induced VSMCs model. We used MEG3 knockout, miR-361-5p mimic and inhibitor to further explore the relationship of these factors. We found that ABCA1 is a direct target of miR-361-5p, moreover, lncRNA MEG3 suppressed VSMCs proliferation and promoted apoptosis by modulating miR-361-5p. Taken together, this story demonstrated that lncRNA MEG3-derived miR-361-5p regulate vascular smooth muscle cells proliferation and apoptosis by targeting ABCA1.
There are several limitations in our study. We used downexpression system, which may not truly reflect activation of endogenous factors. In addition, our study only used in vitro model, there is still a distance to reach the animal even human physiological and pathological condition. In future, we are looking forward to more valid and better-designed in vivo or even clinical experiments.
Conclusion
In conclusion, our study identified that lncRNA MEG3 acts as a regulator in the arrest of VSMC proliferation and apoptosis. LncRNA MEG3 may function as an endogenous sponge of miR-361-5p and further regulate ABCA1 expression. The exploitation of lncRNA MEG3 may lead to the development of new therapeutics for atherosclerosis.
Acknowledgements
This study was supported by Tianjin First Center Hospital.
Disclosure of conflict of interest
None.
References
- 1.Rosenberg MA, Lopez FL, Buzkova P, Adabag S, Chen LY, Sotoodehnia N, Kronmal RA, Siscovick DS, Alonso A, Buxton A, Folsom AR, Mukamal KJ. Height and risk of sudden cardiac death: the atherosclerosis risk in communities and cardiovascular health studies. Ann Epidemiol. 2014;24:174–179. doi: 10.1016/j.annepidem.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal SK, Heiss G, Rautaharju PM, Shahar E, Massing MW, Simpson RJ Jr. Premature ventricular complexes and the risk of incident stroke: the atherosclerosis risk in communities (ARIC) study. Stroke. 2010;41:588–593. doi: 10.1161/STROKEAHA.109.567800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Mohanty P, Bhatnagar S. Integrative analysis of ocular complications in atherosclerosis unveils pathway convergence and crosstalk. J Recept Signal Transduct Res. 2015;35:149–164. doi: 10.3109/10799893.2014.942462. [DOI] [PubMed] [Google Scholar]
- 4.Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Uryga AK, Reinhold J, Figg N, Baker L, Finigan A, Gray K, Kumar S, Clarke M, Bennett M. Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation. 2015;132:1909–1919. doi: 10.1161/CIRCULATIONAHA.115.016457. [DOI] [PubMed] [Google Scholar]
- 6.Johnson JL. Emerging regulators of vascular smooth muscle cell function in the development and progression of atherosclerosis. Cardiovasc Res. 2014;103:452–460. doi: 10.1093/cvr/cvu171. [DOI] [PubMed] [Google Scholar]
- 7.Chiong M, Morales P, Torres G, Gutierrez T, Garcia L, Ibacache M, Michea L. Influence of glucose metabolism on vascular smooth muscle cell proliferation. Vasa. 2013;42:8–16. doi: 10.1024/0301-1526/a000243. [DOI] [PubMed] [Google Scholar]
- 8.Wang P, Xu TY, Guan YF, Zhao Y, Li ZY, Lan XH, Wang X, Yang PY, Kang ZM, Vanhoutte PM, Miao CY. Vascular smooth muscle cell apoptosis is an early trigger for hypothyroid atherosclerosis. Cardiovasc Res. 2014;102:448–459. doi: 10.1093/cvr/cvu056. [DOI] [PubMed] [Google Scholar]
- 9.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 10.Ping S, Li Y, Liu S, Zhang Z, Wang J, Zhou Y, Liu K, Huang J, Chen D, Wang J, Li C. Simultaneous increases in proliferation and apoptosis of vascular smooth muscle cells accelerate diabetic mouse venous atherosclerosis. PLoS One. 2015;10:e0141375. doi: 10.1371/journal.pone.0141375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnsson P, Lipovich L, Grander D, Morris KV. Evolutionary conservation of long non-coding RNAs; sequence, structure, function. Biochim Biophys Acta. 2014;1840:1063–1071. doi: 10.1016/j.bbagen.2013.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu X, Li Z. Long non-coding RNA HOTAIR: a novel oncogene (review) Mol Med Rep. 2015;12:5611–5618. doi: 10.3892/mmr.2015.4161. [DOI] [PubMed] [Google Scholar]
- 13.Han P, Chang CP. Long non-coding RNA and chromatin remodeling. RNA Biol. 2015;12:1094–1098. doi: 10.1080/15476286.2015.1063770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dey BK, Mueller AC, Dutta A. Long non-coding RNAs as emerging regulators of differentiation, development, and disease. Transcription. 2014;5:e944014. doi: 10.4161/21541272.2014.944014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang F, Xue X, Zheng L, Bi J, Zhou Y, Zhi K, Gu Y, Fang G. Long non-coding RNA GHET1 promotes gastric carcinoma cell proliferation by increasing c-Myc mRNA stability. FEBS J. 2014;281:802–813. doi: 10.1111/febs.12625. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Song JH, Cheng Y, Wu W, Bhagat T, Yu Y, Abraham JM, Ibrahim S, Ravich W, Roland BC, Khashab M, Singh VK, Shin EJ, Yang X, Verma AK, Meltzer SJ, Mori Y. Long non-coding RNA HNF1A-AS1 regulates proliferation and migration in oesophageal adenocarcinoma cells. Gut. 2014;63:881–890. doi: 10.1136/gutjnl-2013-305266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeOcesano-Pereira C, Amaral MS, Parreira KS, Ayupe AC, Jacysyn JF, Amarante-Mendes GP, Reis EM, Verjovski-Almeida S. Long non-coding RNA INXS is a critical mediator of BCL-XS induced apoptosis. Nucleic Acids Res. 2014;42:8343–8355. doi: 10.1093/nar/gku561. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Shan K, Jiang Q, Wang XQ, Wang YN, Yang H, Yao MD, Liu C, Li XM, Yao J, Liu B, Zhang YY, J Y, Yan B. Role of long non-coding RNA-RNCR3 in atherosclerosis-related vascular dysfunction. Cell Death Dis. 2016;7:e2248. doi: 10.1038/cddis.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang YN, Shan K, Yao MD, Yao J, Wang JJ, Li X, Liu B, Zhang YY, Ji Y, Jiang Q, Yan B. Long noncoding RNA-GAS5: a novel regulator of hypertension-induced vascular remodeling. Hypertension. 2016;68:736–748. doi: 10.1161/HYPERTENSIONAHA.116.07259. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J, Zhang W, Lin M, Wu W, Jiang P, Tou E, Xue M, Richards A, Jourd’heuil D, Asif A, Zheng D, Singer HA, Miano JM, Long X. MYOSLID is a novel serum response factor-dependent long noncoding RNA that amplifies the vascular smooth muscle differentiation program. Arterioscler Thromb Vasc Biol. 2016;36:2088–2099. doi: 10.1161/ATVBAHA.116.307879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ergun S, Oztuzcu S. Oncocers: ceRNA-mediated cross-talk by sponging miRNAs in oncogenic pathways. Tumour Biol. 2015;36:3129–3136. doi: 10.1007/s13277-015-3346-x. [DOI] [PubMed] [Google Scholar]
- 22.Song X, Shan D, Chen J, Jing Q. miRNAs and lncRNAs in vascular injury and remodeling. Sci China Life Sci. 2014;57:826–835. doi: 10.1007/s11427-014-4698-y. [DOI] [PubMed] [Google Scholar]
- 23.Leung A, Trac C, Jin W, Lanting L, Akbany A, Saetrom P, Schones DE, Natarajan R. Novel long noncoding RNAs are regulated by angiotensin II in vascular smooth muscle cells. Circ Res. 2013;113:266–278. doi: 10.1161/CIRCRESAHA.112.300849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian S, Yuan Y, Li Z, Gao M, Lu Y, Gao H. LncRNA UCA1 sponges miR-26a to regulate the migration and proliferation of vascular smooth muscle cells. Gene. 2018;673:159–166. doi: 10.1016/j.gene.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Yang Z, Wang WE, Zhang Q. CIAPIN1 siRNA inhibits proliferation, migration and promotes apoptosis of VSMCs by regulating Bcl-2 and Bax. Curr Neurovasc Res. 2013;10:4–10. doi: 10.2174/156720213804805909. [DOI] [PubMed] [Google Scholar]
- 26.Yu H, Clarke MC, Figg N, Littlewood TD, Bennett MR. Smooth muscle cell apoptosis promotes vessel remodeling and repair via activation of cell migration, proliferation, and collagen synthesis. Arterioscler Thromb Vasc Biol. 2011;31:2402–2409. doi: 10.1161/ATVBAHA.111.235622. [DOI] [PubMed] [Google Scholar]
- 27.Zhang F, Ren X, Zhao M, Zhou B, Han Y. Angiotensin-(1-7) abrogates angiotensin II-induced proliferation, migration and inflammation in VSMCs through inactivation of ROS-mediated PI3K/Akt and MAPK/ERK signaling pathways. Sci Rep. 2016;6:34621. doi: 10.1038/srep34621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotllan N, Wanschel AC, Fernandez-Hernando A, Salerno AG, Offermanns S, Sessa WC, Fernandez-Hernando C. Genetic evidence supports a major role for Akt1 in VSMCs during atherogenesis. Circ Res. 2015;116:1744–1752. doi: 10.1161/CIRCRESAHA.116.305895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mondal T, Subhash S, Vaid R, Enroth S, Uday S, Reinius B, Mitra S, Mohammed A, James AR, Hoberg E, Moustakas A, Gyllensten U, Jones SJ, Gustafsson CM, Sims AH, Westerlund F, Gorab E, Kanduri C. MEG3 long noncoding RNA regulates the TGF-beta pathway genes through formation of RNA-DNA triplex structures. Nat Commun. 2015;6:7743. doi: 10.1038/ncomms8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng W, Si S, Zhang Q, Li C, Zhao F, Wang F, Yu J, Ma R. Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate gastric cancer progression. J Exp Clin Cancer Res. 2015;34:79. doi: 10.1186/s13046-015-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Zou Y, Wang W, Zuo Q, Jiang Z, Sun M, De W, Sun L. Down-regulated long non-coding RNA MEG3 and its effect on promoting apoptosis and suppressing migration of trophoblast cells. J Cell Biochem. 2015;116:542–550. doi: 10.1002/jcb.25004. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Yan G, Zhang Y, Jia X, Bu P. Long non-coding RNA MEG3 suppresses migration and invasion of thyroid carcinoma by targeting of Rac1. Neoplasma. 2015;62:541–549. doi: 10.4149/neo_2015_065. [DOI] [PubMed] [Google Scholar]
- 33.Zhu J, Liu S, Ye F, Shen Y, Tie Y, Zhu J, Wei L, Jin Y, Fu H, Wu Y, Zheng X. Long noncoding RNA MEG3 interacts with p53 protein and regulates partial p53 target genes in hepatoma cells. PLoS One. 2015;10:e0139790. doi: 10.1371/journal.pone.0139790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu KH, Li W, Liu XH, Sun M, Zhang ML, Wu WQ, Xie WP, Hou YY. Long non-coding RNA MEG3 inhibits NSCLC cells proliferation and induces apoptosis by affecting p53 expression. BMC Cancer. 2013;13:461. doi: 10.1186/1471-2407-13-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Zhong Y, Wang Y, Zhang X, Batista DL, Gejman R, Ansell PJ, Zhao J, Weng C, Klibanski A. Activation of p53 by MEG3 non-coding RNA. J Biol Chem. 2007;282:24731–24742. doi: 10.1074/jbc.M702029200. [DOI] [PubMed] [Google Scholar]
- 36.Li P, Gao Y, Li J, Zhou Y, Yuan J, Guan H, Yao P. LncRNA MEG3 repressed malignant melanoma progression via inactivating wnt signaling pathway. J Cell Biochem. 2018;119:7498–7505. doi: 10.1002/jcb.27061. [DOI] [PubMed] [Google Scholar]
- 37.Liang Z, Chi YJ, Lin GQ, Xiao LF, Su GL, Yang LM. LncRNA MEG3 participates in neuronal cell injury induced by subarachnoid hemorrhage via inhibiting the Pi3k/Akt pathway. Eur Rev Med Pharmacol Sci. 2018;22:2824–2831. doi: 10.26355/eurrev_201805_14983. [DOI] [PubMed] [Google Scholar]
- 38.Liu Z, Wu C, Xie N, Wang P. Long non-coding RNA MEG3 inhibits the proliferation and metastasis of oral squamous cell carcinoma by regulating the WNT/beta-catenin signaling pathway. Oncol Lett. 2017;14:4053–4058. doi: 10.3892/ol.2017.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu W, Liu X, Luo M, Liu X, Luo Q, Tao H, Wu D, Lu S, Jin J, Zhao Y, Zou L. dNK derived IFN-gamma mediates VSMC migration and apoptosis via the induction of LncRNA MEG3: a role in uterovascular transformation. Placenta. 2017;50:32–39. doi: 10.1016/j.placenta.2016.12.023. [DOI] [PubMed] [Google Scholar]