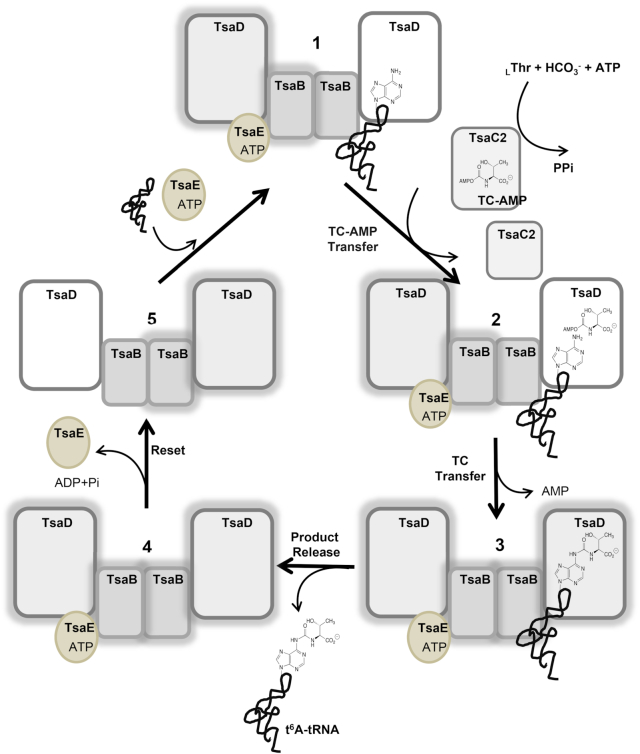
Figure 1.
The bacterial t6A biosynthesis cycle, shown here for T. maritima. tRNA and ATP-bound TsaE bind to separate sites on the TmTsaB2D2 heterocomplex (1). TC–AMP, synthesized by TsaC2, is then delivered to the active site of the tRNA-bound TsaD subunit (2), and the threonylcarbamoyl (TC) moiety is transferred to A37 of tRNA, forming t6A (3). Following dissociation of the t6A-modified tRNA from the complex (4), ATP hydrolysis resets the system to the initial state and TsaE is released (5), thus allowing the next substrate tRNA to bind for the next cycle. The two halves of the complex are reset and utilized in alternating cycles. Halos indicate a pre-‘reset’ state for a given half.