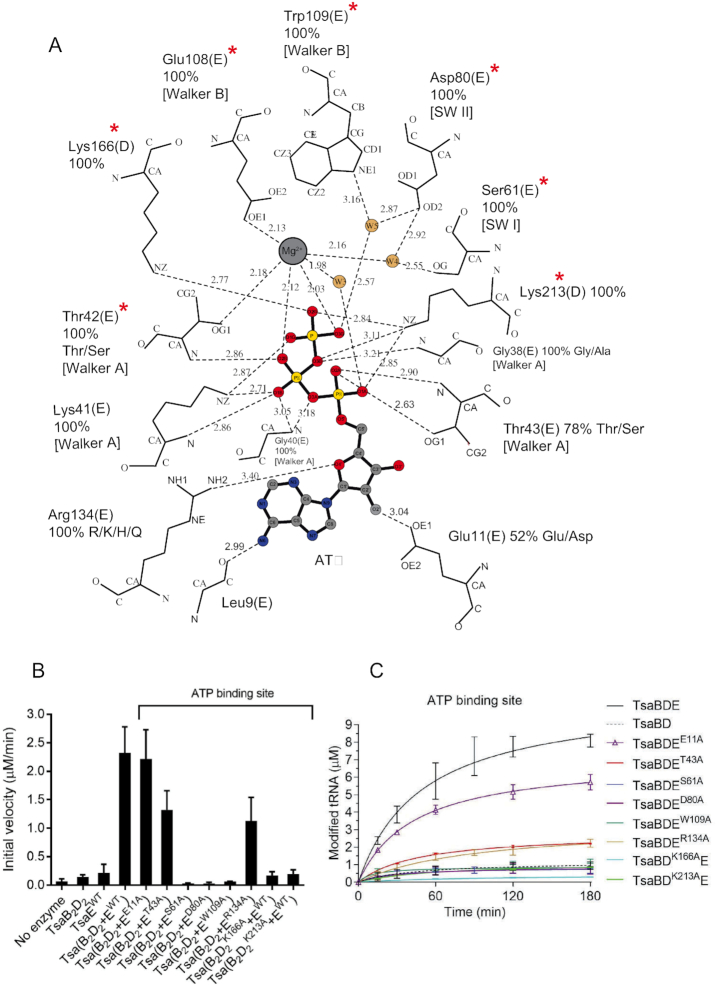
Figure 4.
Interactions and mutagenesis of the ATPase site. (A) Schematic of the interactions with Mg2+-ATP bound in the ATPase site at the interface between the TsaB and TsaD subunits, as seen in the crystal structure of TmTsaB2D2E2. The nucleotide, Mg2+ and water molecules are shown in ball-and-stick representation and colored. Protein residues are labeled, their percent conservation in 250 inspected sequences, and the subunits to which they belong are indicated in parentheses. Where relevant for TsaE residues, the conserved TsaE motif is indicated in brackets. Residues required for multiple-turnover t6A synthesis are indicated with red asterisks. Hydrogen bonds are shown as dashed line. The figure was made in LIG-PLOT (45). (B) Effect of mutations in the ATPase site on the ATP hydrolysis activity of the TC-transfer complex. Shown are the initial velocities of ATP hydrolysis reactions catalyzed by equimolar mixtures of pre-isolated TmTsaB2D2 and TmTsaE harboring ATPase-site mutations either in the TsaE or the TsaD subunit. Data represent averages of duplicate measurements from three independent reactions. (C) Effect of mutations in the ATPase site on the turnover of the t6A biosynthesis reaction, assessed using the time-course radiochemical t6A assay measuring the incorporation of [14C]-L-threonine into tRNA. Assays contained either mutant TmTsaE or mutant TmTsaD harboring mutations in the ATPase site, and the other three wild-type Tsa proteins. Multiple-turnover activity in the presence of all wild-type proteins (solid black line) and single-turnover activity in the absence of TmTsaE (dashed black line) are also shown. Data represent duplicate measurements from at least three independent reactions.