Figure 6.
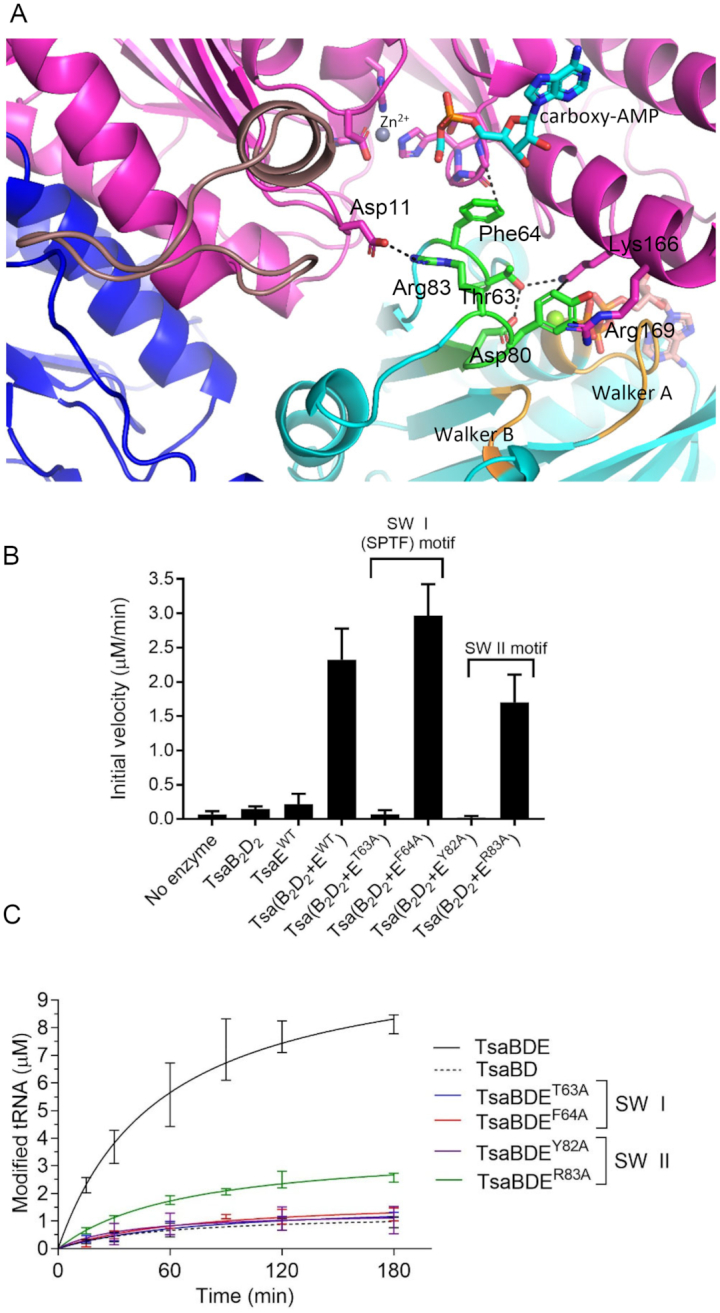
Role of Switch I and Switch II residues of TmTsaE in signal transduction between the ATPase site and TC-transfer site. (A) Interactions of Thr63 and Phe64 in Switch I loop, and Tyr82 and Arg83 in Switch II loop of TsaE and their locations relative to the bound nucleotides in the ATPase and TC-transfer sites. (B) Effect of mutations in Switch I and Switch II motifs on the ATPase activity of the TC-transfer complex. Shown are initial velocities of ATP hydrolysis reactions catalyzed by an equimolar mixture of pre-isolated wild-type TmTsaB2D2 and mutant TmTsaE proteins harboring mutations in Switch I or Switch II. Data represent averages of duplicate measurements from three independent reactions. (C) Effect of mutations in Switch I and Switch II motifs on the turnover of the t6A biosynthesis reaction, assessed using the time-course radiochemical t6A assay measuring the incorporation of [14C]-threonine into tRNAThrCGU. Assays contained mutant TmTsaE and the other three wild-type Tsa proteins (colored lines). Multiple-turnover activity catalyzed by a mixture of all wild-type proteins (solid black line), and single-turnover activity catalyzed in the absence of TmTsaE (dashed black line) are shown. Data represent duplicate measurements from three independent reactions.
