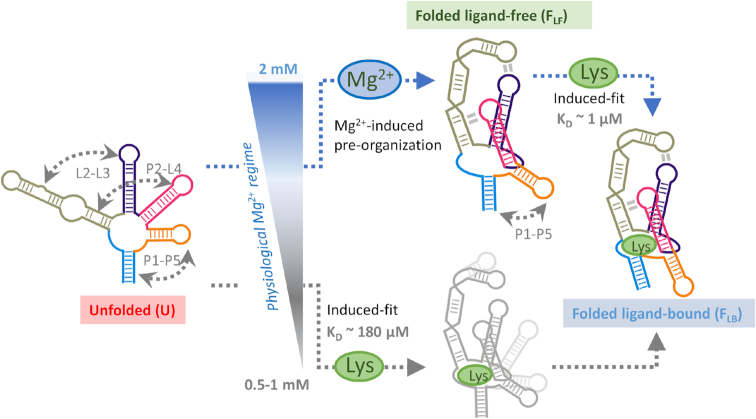
Figure 6.
The folding and ligand-binding mechanism of the lysine aptamer is controlled by a narrow change in the physiological concentration of Mg2+ ions. Schematic of the interplay between folding and induced-fit ligand binding in the lysC aptamer, emphasising the structural changes taking place within a very narrow Mg2+ concentration window and the alternative folding routes predominant at each condition. At the low end of the physiological Mg2+ concentration range (<1 mM), the aptamer remains unstructured, and the encounter complex needs to form most tertiary contacts with the assistance of the ligand (lower route). An increase of just 1 mM in the concentration of Mg2+ ions is enough to shift the aptamer to a tertiary pre-organised structure (upper route). The encounter complex evolves into the native state by shortening the P1–P5 distance and closing the ligand-binding pocket. The lysine aptamer uses a dual-input signalling model to switch between a single-step, poor-affinity pathway at low concentrations of Mg2+ ions to a high-affinity, two-step mechanism at high physiological concentrations.