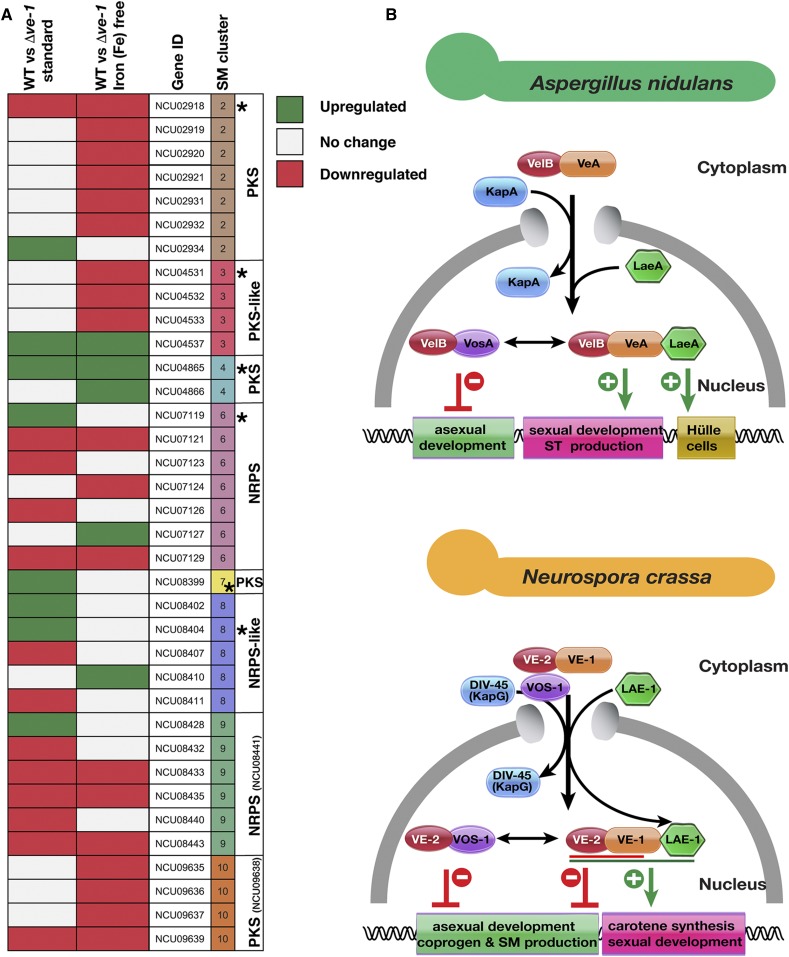
Figure 8.
Expression of secondary metabolite genes and comparative model of the velvet complexes in A. nidulans and N. crassa. (A) Expression of eight putative secondary metabolite gene clusters of Neurospora in liquid 48 hr cultures under standard or iron starvation conditions in an Δve-1 strain in comparison to WT. PKS: Polyketide synthase, NRPS: Nonribosomal peptide synthase. SM cluster numbers, and the “backbone” genes (with NCU numbers indicated) in the clusters are identified. Backbone genes were indicated with an asterisk. Expression of some backbone genes did not change (those without the asterisks) (see Table S13). (B) Velvet complex has been studied mostly in A. nidulans (upper panel). The VelB-VeA heterodimer, formed by the two velvet family proteins (represented by red and orange spherical shapes), enters into the nucleus with help of importin α (KapA, Blue). In the nucleus VelB-VeA form a heterodimer with the methyltransferase LaeA. This complex has several functions; the VeA-VelB part of the complex is mainly responsible for sexual development and SM production (shown as green lines). In addition, LaeA has a particular function on formation of protective hülle cells required for protection and nursing of growing fruit bodies. In addition to its role in the velvet complex, VelB is also a part of heterodimeric complex formed with VosA, which activates spore viability, trehalose accumulation (green lines) and also represses asexual conidiation (red lines). In N. crassa, the situation is similar. The VE-1/VE-2 or VE-2/VOS-1 heterodimer enters into nucleus, presumably with help of importin DIV-45 (KAP114p ortholog) since DIV-45 was found in purifications of both VE-2 and VOS-1. The VE-2/VE-1 heterodimer is a major player to repress asexual conidiation and SM production including coprogen (red line) and to activate carotene biosynthesis (green line). The VE-1/VE-2/LAE-1 complex is required for maximum protoperithecia formation (sexual development). The VE-2/VOS-1 heterodimer presumably represses conidiation and hyphal growth.