Abstract
PURPOSE:
To study whether institutional clinical trial accrual volume affects clinical outcomes of younger (age less than 61 years) patients with acute myeloid leukemia.
PATIENTS AND METHODS:
We investigated the impact of clinical trial accrual on response rates, early mortality and survival in patients with AML enrolled between 2002 and 2009 into two parallel cooperative group clinical trials SWOG S0106/ECOG-ACRIN E1900. Institutions were classified as low- (LAIs) (≤ 9 enrolled patients) or high-accruing institutions (HAIs) (≥ 10 enrolled patients). Fisher’s exact text and logistic regression analysis were used to analyze the response and early mortality rates. The effect of accrual volume on survival was analyzed by log-rank tests and Cox regression models.
RESULTS:
A total of 1252 patients from 152 institutions were included in the final analyses. The median clinical trial registrations in HAIs was 19 patients (range, 10 to 92) versus 3 (range, 1 to 9) patients in LAIs. In multivariate analyses, HAIs, as a quantitative covariate, was associated with improved complete remission rates (odds ratio (OR) 1.08, p = 0.0051),, but no improvement median overall survival (HR 0.97, p = 0.065) or median event-free (hazard ratio (HR) 0.97, p = 0.05). Early mortality rates were similar between cohorts and academic affiliation had no impact on response rates or survival.
CONCLUSION:
Clinical trial accrual volume, had an independent, albeit modest, impact on complete remission rates, but not on overall survival and event-free in younger patients with AML.
Keywords: AML, Clinical trial, volume, outcomes, Leukemia, population-based
INTRODUCTION
The correlation between increased hospital volume and improved outcomes is a remarkably consistent observation in studies of surgical specialties, including operations and interventional procedures, and nonsurgical hospital-based care, such as treatment of congestive heart failure, mechanical ventilation, and intensive care.1,2,3,4 In oncology, similar volume-outcome correlations have been reported in patients with head and neck and lung cancer in addition to patients undergoing allogeneic stem cell transplant (alloHCT) for chronic myeloid leukemia.5,6,7 In patients with acute myeloid leukemia (AML), only limited data are available showing that high volume hospitals observed lower mortality rates following inpatient chemotherapy compared to low volume hospitals.8 This study, however, was limited by heterogeneous cohorts of patients, lack of information regarding chemotherapy regimen used or stage of the disease being treated (induction versus consolidation or in remission versus relapsed/refractory) and absence of critical prognostic factors in AML, such as baseline white blood cell and platelet count, performance status at diagnosis and cytogenetic information.
Principles of good clinical practices are critical requirements to the conduct of research involving human subjects and were developed to ensure that study subjects receive safe, high-quality, protocol-driven care from highly trained research personnel overseen by experienced and well-informed investigators. The same elements required for cancer centers to participate in clinical research protocols could also translate into beneficial changes in the hospital environment that affects outcomes of care for patients treated outside the trial setting.9,10,11 Therefore, it is possible that institutional clinical trial accrual could, at least partially, serve as a surrogate for clinical expertise and impact short- and long-term outcomes in AML. In fact, these effects may be particular relevant in patients with AML where clinical trial accrual rates routinely exceed accrual rates observed in solid tumor clinical trials and patients are frequently cared in academic centers with emphasis on clinical research.12,13
To address the effect of hospital volume on outcomes in a well-defined and homogenous cohort of AML patients, we examined whether institutional clinical trial accrual volume, used as a surrogate for institutional experience, was associated with induction mortality or survival for younger (≤ 60 years) patients with previously untreated AML enrolled onto two prospective, multicenter, randomized controlled trials conducted by SWOG (formerly known as Southwest Oncology Group) and Eastern Cooperative Oncology Group-American College of Radiology Imaging Network (ECOG-ACRIN).
METHODS
Study design and selection of datasets
We analyzed individual patient data from two randomized controlled trials activated by the National Cancer Institute cooperative group trials to adult patients with AML between December 2002 and August 2009. These results of these studies have been described.14,15,16,17 In brief, patients had AML according to the World Health Organization criterion (≥20% blasts), aged 17 to 60 years, with adequate performance status and organ function. Patients with acute promyelocytic leukemia (M3 AML) were not eligible. All patients provided written informed consent in accordance with local policies, federal regulations, and the Declaration of Helsinki. These trials were registered with www.clinicaltrials.gov as #NCT00085709 and NCT00049517.
Study design and treatment groups
ECOG-ACRIN 1900
Patients with untreated AML were randomized to receive either 45 mg/m2 or 90 mg/m2 of intravenous daunorubicin daily for 3 days, together with intravenous cytarabine (100 mg per square meter per day) continuously for 7 days from December 2002 through November 2008. A second induction course with daunorubicin 45 mg/m2 could be given for persistent disease. Post-remission therapy options included alloHCT or autologous stem-cell transplantation following two cycles of high-dose cytarabine therapy, based on the risk of relapse. Patients who underwent autologous stem-cell transplantation were randomized to receive a single dose of gemtuzumab ozogamicin prior to transplant. Inclusion of patients with a history of an antecedent hematologic disorder within 6 months of AML diagnosis was permitted.
SWOG S0106
Between August 2004 through August 2009, patients were randomized between 2 induction regimens: either DA (daunorubicin 45 mg/m2 days 1 – 3 plus cytarabine 100 mg/m2 days 1 – 7) plus GO (gemtuzumab ozogamicin 6 mg/m2 on day 4); or DA (daunorubicin 60 mg/m2 days 1 – 3 plus cytarabine 100 mg/m2 on days 1 – 7). A second course using the DA regimen was allowed for patients with residual AML. Post-remission therapy for patients in remission included 3 courses of consolidation therapy with cytarabine 3 g/m2 every 12 hours on days 1, 3, and 5. After consolidation therapy, patients were randomized between GO (5 mg/m2, 3 doses at least 28 days apart) vs observation. Patients with AML arising from a prior hematological malignancy were ineligible for this study.
Treatment outcomes
Complete remission (CR) was defined according to the International Working Group Guidelines.18 Early mortality (EM) was defined as death within 28 days of initiating therapy.19 Event-free survival (EFS) was measured from date of randomization to the first of date of protocol therapy without a CR, relapse from CR, or death from any cause, with observations censored at the day of last contact for patients last known to be alive without report of relapse. Overall survival (OS) was measured for all patients from the day of initial randomization until death from any cause, with censoring at the day of last contact for patients last known to be alive. Relapse-free survival (RFS) was measured for patients who achieved CR from the date of CR until the first of relapse or death from any cause, with observations censored at the day of last contact for patients last known to be alive without report of relapse.
Statistical considerations
Our primary objective was to analyze the association between institutional accrual volume and outcomes. As a surrogate for institutional expertise, we used institutional accrual volume to two recent AML clinical trials conducted by SWOG and ECOG-ACRIN during the 7-year period. Data were collected and evaluated according to the standard practices of SWOG and ECOG-ACRIN. Median institutional accrual to S0106 was used to define the separate accrual categories: institutions that registered 10 or more eligible patients were denoted hereafter High Accruing Institutions (HAIs) and institutions that registered 9 or fewer eligible patients were denoted Low Accruing Institutions (LAIs). Hospital volume was analyzed quantitatively (number of eligible patients registered to the trial from same institution) and as a binary covariate (10 or more patients versus 9 or fewer patients). To avoid confounding of outcomes by study and treatment arm, analyses were stratified by study (SWOG versus ECOG-ACRIN) and treatment arm. Fisher’s exact text and logistic regression analysis were used to analyze the effects of treatment group and other covariates on CR rates and early death. Log-rank tests and Cox regression were used to analyze OS, RFS, and EFS. Academic affiliation was defined as those institutions with Accreditation Council for Graduate Medical Education (ACGME) training programs for hematology and/or medical oncology.
RESULTS
Study Population and comparison between SWOG and ECOG trials
There were 1252 patients included in the following analyses. Patients treated in the SWOG trial had higher CR rates (70% versus 65%, p=0.05). In addition, longer median OS (47 months versus 21 months, p<0.0001) was observed for patients treated on the SWOG trial. These differences remained significant when patients with secondary AML (n=22) enrolled on the ECOG-ACRIN trial were excluded from the analysis (p < 0.001). A histogram depicting institutional volume is presented on Supplemental Figure 1. Among the 152 institutions participating on these studies, a total of 846 (68%) patients (median of 19 patients (range, 10 to 92) were registered by the 34 (22%) HAIs. LAIs (n=118) registered a median of 3 (range, 1 to 9) patients on trial. Differences in the distribution of HAIs were observed in patients enrolled in the two clinical trials. For the ECOG-ACRIN E1900 clinical trial, approximately 40% of all patients registered were enrolled in one of four institutions. The four highest accruing institutions in the SWOG-led S0106 trial enrolled only 20% of all patients. Patients at HAIs were more commonly males and registered at academic centers. Time to initiation of therapy was similar between HAIs and LAIs (p= 0.23). Other baseline characteristics for subjects included in our analyses are summarized in Table 1.
Table 1.
Patient and Tumor Characteristics by Accrual Volume
| Variable | LAIs (n = 406) | HAIs (n = 846) | p-value |
|---|---|---|---|
| Age (median, range) | 47 (18,61) | 48 (17,61) | 0.36 |
| WBC at diagnosis (x 109/L, range) |
11 (0,370) | 12 (0,545) | 0.06 |
| Bone Marrow Blasts (% median, range) | 64 (3,100) | 65 (3,100) | 0.25 |
| Female (N, %) | 179 (44) | 425 (50) | 0.046 |
| ECOG Performance Status (N, %) | |||
| 0–1 | 357 (88) | 772 (92) | 0.16 |
| 2–3 | 47 (12) | 69 (8) | |
| Response to Induction Chemotherapy (N, %) | |||
| Complete Remission | 266 (66) | 575 (68) | 0.24 |
| Early Mortality | 15 (4%) | 25 (3%) | 0.42 |
| Institution Affiliation (N, %) | |||
| Non-academic | 266 (66) | 274 (32) | <0.01 |
| Academic | 140 (34) | 572 (68) | |
| Cytogenetic risk (NCCN) | |||
| Favorable | 52 (13) | 114 (13) | 0.12 |
| Intermediate | 161 (41) | 285 (34) | |
| Unfavorable | 73 (18) | 176 (21) | |
| Missing | 116 (29) | 271 (32) | |
Footnotes: LAIs – Low Accruing Institutions, HAIs – High Accruing Institutions, WBC – White blood cell count, ECOG – Eastern Oncology Group.
Early mortality and treatment efficacy
Early mortality (EM) rates were not significantly different between HAIs and LAIs (4% versus 3%, p=0.42). On multivariable analysis, neither the binary nor the quantitative institutional volume covariates were associated with EM rates. In addition, CR rates were not significantly different between HAIs and LAIs (68% versus 66%, p = 0.24). However, in a multivariable logistic regression model, higher accrual volume with institution volume as a quantitative covariate was independently associated with improvement in CR rates (odds ratio (OR) 1.08 (95% confidence interval (CI) 1.02, 1.14), p = 0.0051). (Table 1)
Survival Analyses
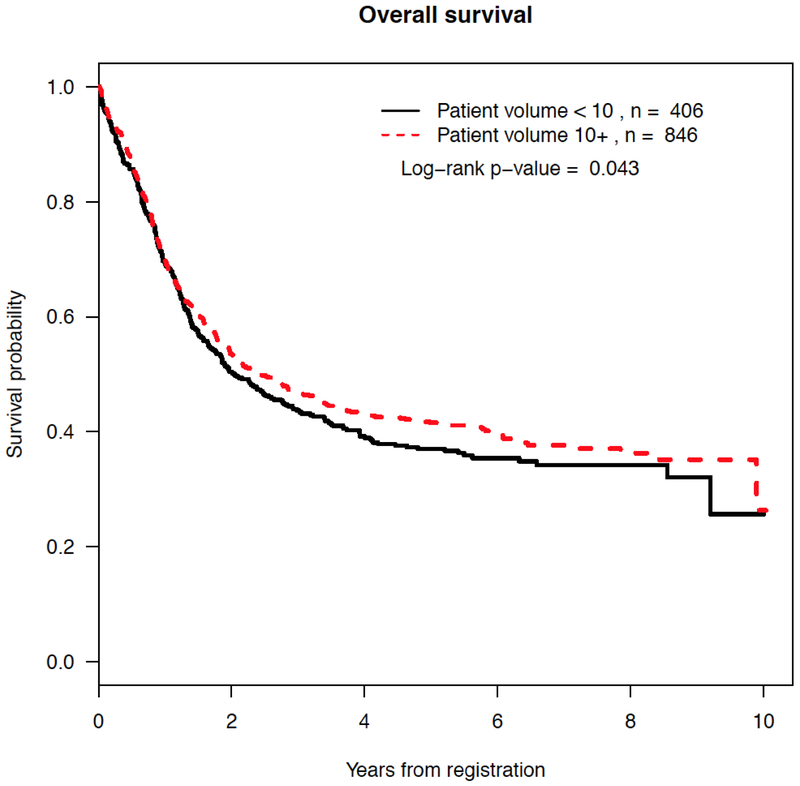
With a median follow-up of 5.6 years among censored patients, patients treated in HAIs had improvement in the overall survival (median 2.4 years for HAIs versus 2.0 years for LAIs, p = 0.043) (Figure 1). No differences in event-free survival (p = 0.098) or relapse-free survival (p = 0.18) were observed between HAIs and LAIs (Supplemental Figure 2). Using institution volume as a quantitative covariate, in Cox regression models favorable and unfavorable karyotype and worse performance status at registration are associated with event-free survival (EFS). Treatment in HAIs (HR 0.97 (95% CI 0.95, 1), p = 0.05) was not independently associated with EFS (Table 2). Treatment at HAIs was not independently associated with relapse-free survival (HR 0.98 (95% CI 0.95, 1.02), p = 0.42) (data not shown) or overall survival (HR 0.97 (95% CI 0.94, 1.0), p = 0.069).
Figure 1.
Kaplan-Meier estimates by accrual volume of overall survival
Table 2.
Cox regression model for overall survival and event free survival using institutional volume as quantitative covariate.
| Covariate | HR (95% CI) | p-value |
|---|---|---|
| Overall Survival (n = 1214) | ||
| Institutional Volume (per 10 patients) | 0.97 (0.94, 1) | 0.069 |
| Age (years) | 1.02 (1.01, 1.02) | <0.01 |
| WBC (x 103) | 1.18 (1.02, 1.36) | 0.022 |
| Bone Marrow Blasts (10%) | 1.03 (1., 1.06) | 0.063 |
| Platelets (x 103) | 1.01 (0.99, 1.03) | 0.25 |
| Male gender (ref = female) | 1.1 (0.95, 1.27) | 0.21 |
| PS 2–4 (ref = PS 0–1) | 1.61 (1.27, 2.04) | <0.01 |
| Favorable risk (ref = intermediate) | 0.53 (0.4, 07) | <0.001 |
| Unfavorable risk(ref = intermediate) | 1.9 (1.57, 2.3) | <0.001 |
| Missing cytogenetic information | 0.95 (0.8, 1.15) | 0.62 |
| Event-Free Survival (n= 1212) | ||
| Institutional Volume (per 10 patients) | 0.97 (0.95, 1) | 0.05 |
| Age (years) | 1.01 (1, 1.01) | 0.1 |
| WBC (x 103) | 1.08 (0.94, 1.24) | 0.26 |
| Bone Marrow Blasts (10%) | 1.02 (0.99, 1.05) | 0.25 |
| Platelets (x 103) | 1.0 (0.99, 1.02) | 0.61 |
| Male gender (ref = female) | 1.07 (0.93, 1.22) | 0.33 |
| PS 2–4 (ref = PS 0–1) | 1.3 (1.04, 1.63) | 0.02 |
| Favorable risk (ref = intermediate) | 0.55 (0.43–0.71) | <0.001 |
| Unfavorable risk(ref = intermediate) | 1.48 (1.24, 1.77) | <0.001 |
| Missing cytogenetic information | 1 (0.85, 1.04) | 0.97 |
Abbreviations: ref - reference
Impact of Treatment on Academic Institutions
A greater proportion of patients in the high-volume category were treated at academic institutions (68% versus 34%, p<0.001). However, no significant differences in CR rate (p = 0.36), EFS (p = 0.081), RFS (p = 0.084), or OS (p = 0.74) were observed between subjects treated at academic versus non-academic institutions. Type of institution (academic versus non-academic) was also not significantly associated with any of these outcomes on multivariable analysis and there were no significant interactions between type of institution and quantitative and non-quantitative volume (summaries not shown).
DISCUSSION
In an analysis of two recent US Intergroup AML clinical trials, we observed modest associations between trial accrual volume and CR rates, but no improvement in OS or EFS. Our observations add to evidence demonstrating that experienced care teams likely execute superior treatment plans that translated into an improvement in remission rates, the most intense treatment period in AML induction therapy. Interestingly, early death rates were similar between cohorts. Because academic institutions were significantly more likely to be in the high-volume category, we hypothesized that differences in academic affiliation could explain the discrepancies between in clinical outcomes. Our results failed to demonstrate an association between type of institution and CR, EFS or OS.
Unfortunately, cooperative group sponsored clinical trials in AML do not adequately collect data on type and timing of alloHCT or salvage therapies following relapse. Thus, we cannot evaluate whether treatment in HAIs had an impact on referral to alloHCT, the potential timing of potential transplants or whether alloHCT influenced outcomes for patients included in these analyses. This information is of particular interest in these cohorts given that survival was similar between HAIs and LAIs up to 12 months post randomization. These observations suggest that late relapses or deaths (such as transplant related mortality) may have negatively impacted outcomes in LAIs. In addition, location of post-remission treatment cycles is not captured adequately and it may also impact our observations.
Despite extensive data demonstrating a strong correlation between institutional or procedure volume and improved clinical outcomes in solid-tumors and non-malignant conditions, only limited data have been reported in patients undergoing intensive therapy for AML.20 Our results, demonstrating improved CR rates add modestly to a report demonstrating a positive correlation between decreased mortality in institutions treating a higher volume of patients with AML.8 In addition, our observations validate and extend the recommendations from British Authorities stipulating that AML patients only receive therapy at specialized institutions treating a minimum of 5 patients per year with intensive chemotherapy.21
Our collaborative effort among the leukemia committees of two separate cooperative groups has significant strengths. Our observations result from analyses performed in a homogenous cohort of AML patients that were uniformly treated during a similar time period both at academic and community practices. This novel initiative has rarely been utilized in AML clinical trials and it expanded on the sample size analyzed. However, clear justifications for the improved CR rates observed with higher-accruing institutions remain unclear. First, accrual volume was not associated with RFS, suggesting increased relapse rate is unlikely the cause of worse outcomes. It is possible that better salvage strategies are available in institutions that accrue more patients to such trials. Of interest are the observations that outcomes following alloHCT are influenced by institution volume and partially affected by the experience of the care team.7,22
We cannot entirely exclude a possible contribution of referral bias leading to differences in patients treated at HAIs versus LAIs and hence differences in the reported outcomes. However, we observed no differences in the demographics of patients registered in HAIs versus LAIs, except for the association between HAIs mostly being academic institutions. In addition, we did not find any interactions between institution affiliation and outcomes between institutional category. Finally, participation in these clinical trials may not be a surrogate for clinical expertise in AML,23 as accrual to intergroup trials may not be an indicator of center AML volume. In addition to cooperative group trials, AML patients may be treated off study, on industry sponsored, or on investigator-initiated trials, which may neutralize the impact institutional volume on outcomes for these trials (i.e. institutions may have high volume of AML patients that are not treated in cooperative group trials). In fact, previous studies have shown that AML subjects enrolled into research studies are younger, have better performance status and less comorbidity and lower rates of secondary AML than AML patients excluded from clinical trial participation.24,25
In conclusion, our data demonstrate the value of collaborative efforts among cooperative groups and suggest strategies to improve research and treatment for patients with AML. Our results suggest that clinical trial enrollment volume is associated with a modest improvement in remission rates, but no effect on event-free survival or overall survival.
Supplementary Material
Acknowledgement:
The authors wish to gratefully acknowledge the important contributions of the late Dr. Stephen H. Petersdorf to SWOG and to study S0106.
Funding: This investigation was supported in part by the following grants awarded by the National Cancer Institute of the National Institutes of Health: CA180888, CA180819, CA180816, CA180820 CA180794, CA180791, CA180790, CA180828, CA20319, CA13650, CA73590, CA15488, CA23318, CA66636 CA32102, CA38926, CA21115, CA17145 and in part by Wyeth (Pfizer).
REFERENCES
- 1.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. [DOI] [PubMed] [Google Scholar]
- 2.Hannan EL, Racz M, Ryan TJ, McCallister BD, Johnson LW, Arani DT, Guerci AD, Sosa J, Topol EJ. Coronary Angioplasty Volume-Outcome Relationships for Hospitals and Cardiologists. JAMA. 1997;277(11):892–898. [PubMed] [Google Scholar]
- 3.Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O’Brien CR, Rubenfeld GD. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355(1):41–50. [DOI] [PubMed] [Google Scholar]
- 4.Kizer KW. The volume-outcome conundrum. N Engl J Med. 2003;349(22):2159–61. [DOI] [PubMed] [Google Scholar]
- 5.Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol. 2000: 18, 2327–40. [DOI] [PubMed] [Google Scholar]
- 6.Bach PB, Cramer LD, Schrag D, Downey RJ, Gelfand SE, Begg CB.The Influence of Hospital Volume on Survival after Resection for Lung Cancer. N Engl J Med 2001; 345:181–188. [DOI] [PubMed] [Google Scholar]
- 7.Horowitz MM, Przepiorka D, Champlin RE, Gale RP, Gratwohl A, Herzig RH, Prentice HG, Rimm AA, Ringdén O, Bortin MM. Should HLA-identical sibling bone marrow transplants for leukemia be restricted to large centers? Blood. 1992;79(10):2771–4 [PubMed] [Google Scholar]
- 8.Giri S, Pathak R, Aryal MR, Karmacharya P, Bhatt VR, Martin MG. Impact of hospital volume on outcomes of patients undergoing chemotherapy for acute myeloid leukemia: a matched cohort study. Blood. 2015;125(21):3359–60. [DOI] [PubMed] [Google Scholar]
- 9.Janni W, Kiechle M, Sommer H et al. ADEBAR Study Group, Study participation improves treatment strategies and individual patient care in participating centers. Anticancer Res 2006;26 ((5B)) 3661–3668. [PubMed] [Google Scholar]
- 10 +Braunholtz.DA Edwards SJL, Lilford RJ. Are randomized clinical trials good for us (in the short term)? evidence for a “trial effect.” J Clin Epidemiol 2001;54 (3) 217–224. [DOI] [PubMed] [Google Scholar]
- 11.Du Bois A, Rochon J, Lamparter C, Pfisterer J. Pattern of care and impact of participation in clinical studies on the outcome in ovarian cancer. Int J Gynecol Cancer 2005;15 (2) 183–191. [DOI] [PubMed] [Google Scholar]
- 12.Mengis C, Aebi S, Tobler A, Dahler W, Fey MF. Assessment of differences in patient populations selected for excluded from participation in clinical phase III acute myelogenous leukemia trials. J Clin Oncol. 2003;21(21):3933–3939. [DOI] [PubMed] [Google Scholar]
- 13.Dechartres A, Chevret S, Lambert J, Calvo F, Levy V. Inclusion of patients with acute leukemia in clinical trials: a prospective multicenter survey of 1066 cases. Ann Oncol. 2011;22(1):224–233. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM, Racevskis J, Dewald GW, Ketterling RP, Bennett JM, Rowe JM, Lazarus HM, Tallman MS. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361(13):1249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel JP, Gönen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, Van Vlierberghe P, Dolgalev I, Thomas S, Aminova O, Huberman K, Cheng J, Viale A, Socci ND, Heguy A, Cherry A, Vance G, Higgins RR, Ketterling RP, Gallagher RE, Litzow M, van den Brink MR, Lazarus HM, Rowe JM, Luger S, Ferrando A, Paietta E, Tallman MS, Melnick A, Abdel-Wahab O, Levine RL. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–89. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J, Larson RA, Erba HP, Stiff PJ, Stuart RK, Walter RB, Tallman MS, Stenke L, Appelbaum FR. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121(24):4854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luskin MR, Lee JW, Fernandez HF, Abdel-Wahab O, Bennett JM, Ketterling RP, Lazarus HM, Levine RL, Litzow MR, Paietta EM, Patel JP, Racevskis J, Rowe JM, Tallman MS, Sun Z, Luger SM. Benefit of high-dose daunorubicin in AML induction extends across cytogenetic and molecular groups. Blood. 2016;127(12):1551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD; International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. [DOI] [PubMed] [Google Scholar]
- 19.Walter RB, Othus M, Borthakur G, Ravandi F, Cortes JE, Pierce SA, Appelbaum FR, Kantarjian HA, Estey EH. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. J Clin Oncol. 2011;29(33):4417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson MP, Waters TM, Kaplan EK, McKillop CN, Martin MG. Hospital volume and acute myeloid leukemia mortality in Medicare beneficiaries aged 65 years and older. Blood. 2016;128(6):872–4. [DOI] [PubMed] [Google Scholar]
- 21.British Committee for Standards in Haematology, Milligan DW, Grimwade D, Cullis JO, Bond L, Swirsky D, Craddock C, Kell J, Homewood J, Campbell K, McGinley S, Wheatley K, Jackson G. Guidelines on the management of acute myeloid leukaemia in adults. Br J Haematol. 2006;135(4):450–74. [DOI] [PubMed] [Google Scholar]
- 22.Frassoni F, Labopin M, Powles R, Mary JY, Arcese W, Bacigalupo A, Bunjes D, Gluckman E, Ruutu T, Schaefer UW, Sierra J, Vernant JP, Willemze R, de Witte T, Gorin NC. Effect of centre on outcome of bone-marrow transplantation for acute myeloid leukaemia. Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 2000. April 22;355(9213):1393–8. [DOI] [PubMed] [Google Scholar]
- 23.Stevens JM, Macdougall F, Jenner M, Oakervee H, Cavenagh J, Lister AT. Patterns of recruitment into acute myeloid leukaemia (AML) 15 and outcome for young patients withAML at a single referral centre. Br J Haematol. 2009;145(1):40–4 [DOI] [PubMed] [Google Scholar]
- 24.Mengis C, Aebi S, Tobler A, Dähler W, Fey MF. Assessment of differences in patient populations selected for excluded from participation in clinical phase III acute myelogenous leukemia trials. J Clin Oncol. 2003;21(21):3933–9. [DOI] [PubMed] [Google Scholar]
- 25.Dechartres A, Chevret S, Lambert J, Calvo F, Lévy V. Inclusion of patients with acute leukemia in clinical trials: a prospective multicenter survey of 1066 cases. Ann Oncol. 2011. January;22(1):224–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.