Fig. 1 |. Quorum-sensing circuits.
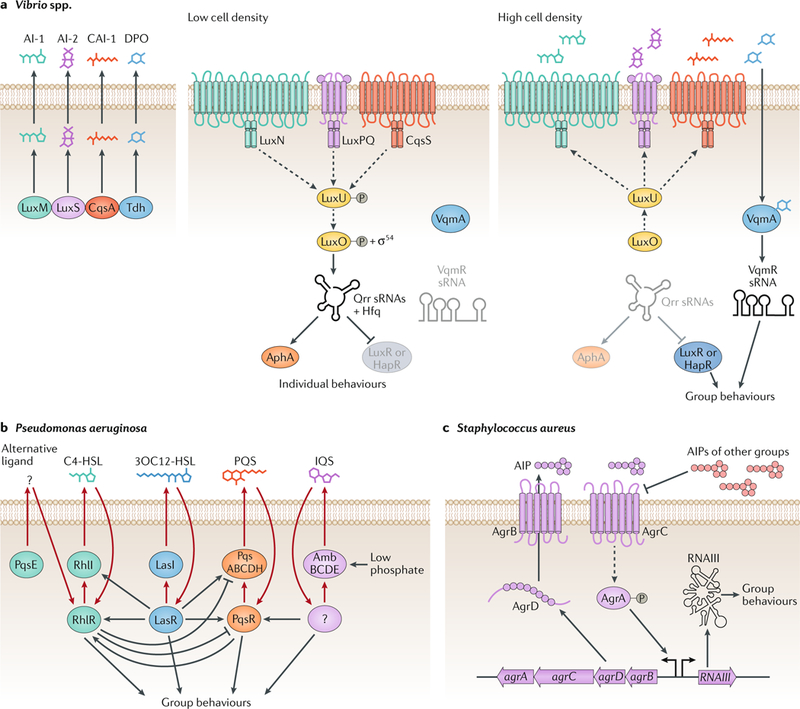
Bacterial quorum sensing relies on networks of autoinducers, autoinducer synthases, partner autoinducer receptors and downstream signal transduction components that convert the information contained in autoinducers into changes in gene expression. a | When Vibrio spp. are at a low cell density, autoinducer levels are low, and their cognate receptors activate a phosphorylation cascade that ultimately results in the activation of the transcription factor AphA, which mediates individual behaviours. By contrast, at high cell density, the synthases LuxM, LuxS, CqsA and Tdh produce high levels of the autoinducers AI-1, AI-2, CAI-1 and DPO, respectively, and the corresponding receptors function as phosphatases. Instead of AphA, LuxR or HapR is produced, which ng loops using LasI and LasR, RhlI, PqsE andmediates group behaviours. b | Pseudomonas aeruginosa employs four interwoven quorum-sensi RhlR, PqsABCDH and PqsR, AmbBCDE and an unknown receptor as the synthases and receptors of the autoinducers 23OC12-HSL, C4-HSL, unknown (PqsE), PQS and IQS, respectively. c | At high cell densities, AgrB from Staphylococcus aureus processes the AgrD precursor peptide and exports the autoinducing peptide AIP, which in turn signals through the AgrC receptor and the downstream transcription factor AgrA. Phosphorylated AgrA induces the production of a regulatory RNA that controls group behaviours. sRNA, small RNA. Dashed lines represent phosphorylation and dephosphorylation. Solid lines represent gene regulation or protein production or small molecule production. Adapted with permission from REF.102, Elsevier.
