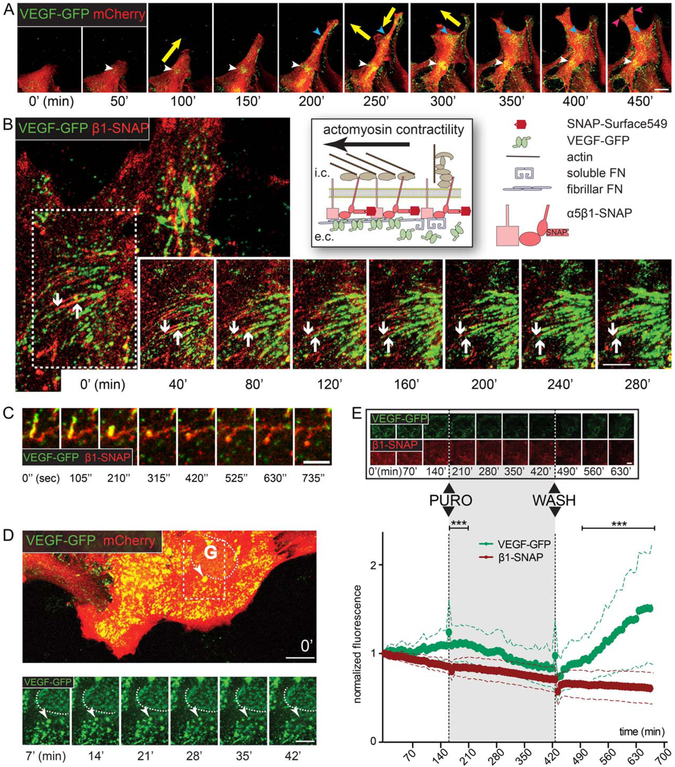
FIGURE 4:
Dynamics of VEGF-GFP in actively assembling ECM. A: Sequential confocal time-lapse images (50 min; Movie 1) of an mCherry – VEGF-GFP cotransduced wound-edge astrocyte demonstrate that VEGF accumulates dynamically at cellular attachment points (colored arrowheads). Note the synchronization between the appearance of VEGF and the changing direction of cell movement (yellow arrows). Bar: 20 μm. B: Schematic of live-stained β1-SNAP integrins in heterodimers and VEGF-GFP during ECM-remodeling on the right. Sequential confocal time-lapse images (40 min intervals; white arrow in Movie 2) of an attachment point of an astrocyte coexpressing VEGF-GFP and β1-SNAP. Downward arrow points at the central end of an adhesion, upward arrow highlights the limit of propagating VEGF-GFP. VEGF-GFP expands inwards along the growing adhesions, and linear VEGF accumulations are formed, demonstrating that astrocytes actively organize growth factor concentrations in their surroundings by linking VEGF-GFP and the remodeled ECM. Bar: 10 μm. C: Cropped image sequence from the same video demonstrates that collapsing adhesions lose their VEGF content (yellow arrows in Movie 2). D: Sequential (7 min) confocal time-lapse images (Movie 3) of VEGF-GFP/mCherry cotransduced astrocytes. Near the Golgi (G, dashed line, its weak intensity is due to intracellular localization) a bright and slow (i.e., surface-bound) VEGF-GFP aggregate (arrowhead) gradually shifts out of focus (i.e., toward intracellular space), depicting the endocytosis of VEGF/ECM complexes. Bars: 20 μm (main) and 10 μm (sequence). E: Sequential confocal time-lapse images (70 min, Movie 5) of an astrocyte coexpressing VEGF-GFP/β1-SNAP show the reversible effect of translation blocking with puromycin (PURO, 10 μg/ml). Bar: 20 μm. Measured whole cell average intensities of such experiments are plotted in time, error bars represent SDs (VEGF-GFP is corrected for bleaching). In contrast to GFP, the intensity of β1-SNAP remained fairly stable during the experiments, indicating its efficient recycling and independence from translation. The substantial differences between the two proteins point out that VEGF accumulation is highly dependent on protein synthesis, and that VEGF is degraded after secretion. 52 cells from 3 experiments, two-way ANOVA with Bonferroni post-test *** P<0.001 (see also Supp. Info. Fig. 2).