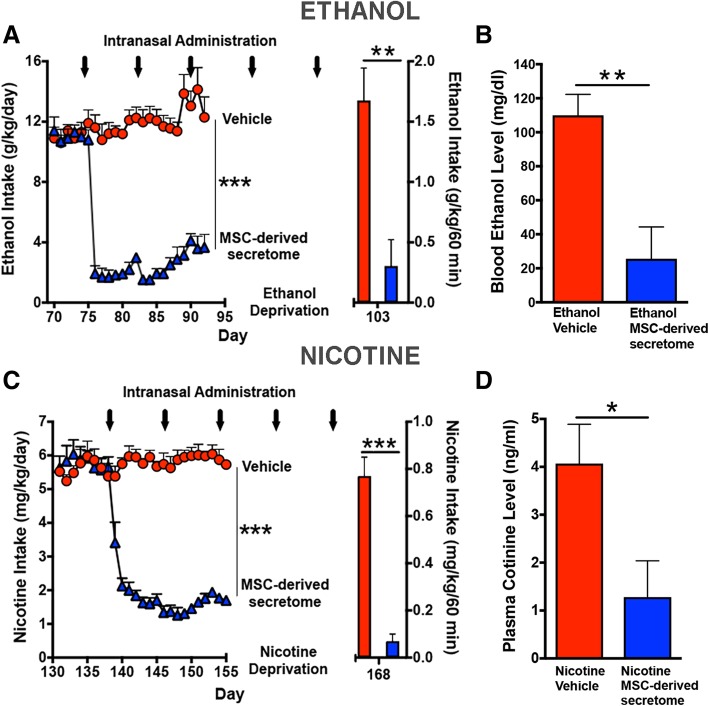
Fig. 2.
Intranasal administration of secretome derived from adipose tissue-derived activated MSCs inhibits chronic ethanol and chronic nicotine intake and blocks post-deprivation binge relapse. Ethanol intake (a, left): red circles show mean ± SEM daily ethanol intake of vehicle intranasal-treated rats (average intake 11.4 ± 0.3 g ethanol/kg body weight; n = 5) that were allowed free-choice access to ethanol (10% and 20% v/v) and water. Arrows indicate the weekly intranasal administration of MSC secretome (160 μl of saline containing 25 μg protein derived from 1 × 106 activated MSCs) or 160 μl of vehicle. Two-way ANOVA (treatment × day) of ethanol intake following 3 intranasal doses of MSC secretome (blue triangles) indicates significant effect of treatment (F1,184 = 1179, p < 0.0001), day (F22,184 = 13.97, p < 0.0001), and significant interaction (Ftreatment × day 22,184 = 18.58, p < 0.001) compared with control rats receiving vehicle (red circles). Bonferroni post-hoc analysis revealed that secretome treatment inhibited ethanol intake (85%) during the 17 days recorded versus vehicle-treated control (p < 0.001; n = 5 per group). a (right) Red bars show ethanol intake after 2 weeks of ethanol deprivation followed by a 60-min period of 10% and 20% ethanol re-access. Rats treated previously with five intranasal secretome doses (blue bar) ingested a significantly lower amount of ethanol (average intake, 0.304 ± 0.22 g ethanol/kg/60 min) than vehicle control animals (red bar, average intake, 1.60 ± 0.27 g ethanol/kg/60 min) (two-tailed t test 3.936, p < 0.001; n = 5 rats/group). b Blood ethanol levels attained immediately after the 60-min ethanol re-access were significantly lower in rats treated with five MSC secretome doses (blue bar) (25.6 ± 18.7 mg/dl, mean ± SEM) than in vehicle control animals (red bar; 110 ± 12 mg/dl) (two-tailed t test = 3.77; p < 0.005; n = 5 rats/group). Nicotine intake (c, left): red circles show the mean (± SEM) daily nicotine intake of vehicle intranasal-treated rats (average 5.6 ± 0.06 mg nicotine/kg; n = 5) that were allowed free-choice access to nicotine (40 mg/l) and water. Two-way ANOVA (treatment × day) of nicotine intake data obtained following 3 intranasal MSC secretome doses (blue triangles) shows a significant effect of treatment (F1,17 = 1232, p < 0.0001), day F24,175 = 22.55, p < 0.0001) and significant interaction (Ftreatment × day 24,175 = 28.92, p < 0.0001) compared with control rats receiving vehicle (red circles). Bonferroni post-hoc test revealed that intranasal secretome induced significant inhibition of nicotine intake (75%) during the 17 days studied versus vehicle-treated rats (p < 0.001; n = 5 rats per group). c (right) Bars show the mean 60-min nicotine consumption upon the nicotine re-access relapse which followed 13 days of nicotine deprivation. Rats treated previously with five intranasal MSC secretome doses (blue bar) ingested a significantly lower amount of nicotine (0.07 ± 0.03 mg/60 min; mean ± SEM) than vehicle control rats (red bar, 0.77 ± 0.08 mg/60 min) (two-tailed t test = 8.193; p < 0.001; n = 5 rats/group). d Blue bar shows that plasma cotinine levels, the main nicotine metabolite, determined immediately after the 60-min nicotine re-access were significantly lower after five intranasal secretome doses (1.28 ± 0.76 ng/ml) than in the vehicle-treated animals (red bar, 4.07 ± 0.82 ng/ml) (two-tailed t test = 2.438; p < 0.04; n = 5 rats/group)