Abstract
Obesity is a major health issue worldwide, and is associated with many diseases including type 2 diabetes. In this study, we evaluated the anti-obesity effects of combinations of two lactic acid bacteria (LAB), Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, and Cinnamomi Ramulus (CR) extract, and explored the mechanism through which they modulate gut microbiota using diet-induced obese mice. Male C57BL/6J mice were randomly divided into five groups that received a high-fat diet (HFD), HFD and LAB (HFD+LAB), HFD and CR extract (HFD+CR), HFD with LAB and CR extract (HFD+LAB+CR), or normal diet for 10 weeks. The mice in the HFD+LAB+CR group showed significant reductions in body weight gain, in particular epididymal fat and liver, blood leptin levels, and an increase in the levels of blood adiponectin. In addition, the LAB and CR extract altered the gut microbiota, mainly increasing the alpha diversity. These results demonstrate that a mixture of two LAB (Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032) and CR extracts alleviate HFD-induced obesity, and has potential of being used as a strategy for the treatment of obesity.
Keywords: anti-obesity, Cinnamomi Ramulus, lactic acid bacteria, gut microbiota
INTRODUCTION
Obesity, a condition in which excess body fat accumulates due to hyperplasia and/or adipocyte hypertrophy (Gan et al., 2017), is a major health issue worldwide and is closely related to metabolic diseases, such as type 2 diabetes, cardiovascular disease, and several types of cancer (Bray, 2000; Leonhardt et al., 1999). According to the World Health Organization, at least 2.8 million people die each year as a consequence of being overweight or obese (Rahman et al., 2017). The two drugs approved by the U.S. Food and Drug Administration, orlistat and sibutramine, are reported to have side effects including gastro- intestinal discomforts, high blood pressure, constipation, headache, heart attack, and insomnia (Heck, 2000; Yun, 2010). Thus, the development of effective natural products for treatment of obesity might evoke interest as they are likely to have minimal side effects.
In recent studies, it has been reported that composition of intestinal microbiota is highly associated with the development of obesity and other related metabolic disorders (Ridaura et al., 2013). The metabolic activities of intestinal microbiota make use of dietary substances ingested by the host; the bacteria help store calories in the host’s adipose tissue and utilize the nutrients for their proliferation, regulating the energy expenditure of the host (DiBaise et al., 2008). Modifying intestinal microbiota could therefore be an option for treating obesity in the future.
Cinnamomi Ramulus (CR), the twig of Cinnamomum cassia Blume, has traditionally been used for treating disorders related to blood circulation and inflammation (Kang and Shin, 2012). Recent studies have demonstrated that CR has various pharmacological effects; for example, CR possesses anti-diabetic (Suppapitiporn et al., 2006) and gastroprotective properties (Jung et al., 2011). Although the associated mechanisms remain unclear, an in vitro study reported that CR reduces lipid accumulation and downregulates expression of adipocyte-differentiation factors in 3T3-L1 preadipocytes (Han et al., 2013).
In our recent study, we reported that oral intake of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 (hereafter, referred to as HY7601 and KY1032, respectively) reduces body fat in mice and humans (Ahn et al., 2015; Park et al., 2013; Yoo et al., 2013), and demonstrated its antiadipogenic effect using 3T3-L1 and HepG2 cells (Jeung et al., 2018). When mice were fed a high-fat diet (HFD) for 8 weeks to induce obesity, followed by a HFD containing HY7601 and KY1032 for an additional 10 weeks, there was a reduction in body weight gain, fat accumulation, and levels of obesity-related factors (Park et al., 2013). Moreover, the accumulation of fat in adipose tissue and liver was reduced when the mice were fed a HFD containing HY7601 and KY1032 (Yoo et al., 2013). An in vitro study indicated that HY7601 and KY1032 reduces adipogenesis in 3T3- L1 and HepG2 cells by regulating transcription factors related to adipogenesis (Jeung et al., 2018). Based on these findings, the present study was conducted to investigate the synergistic effect of feeding HY7601, KY1032, and CR on obesity, and to decipher the mechanism underlying modification of intestinal microbiota in diet-induced obese mice.
MATERIALS AND METHODS
Isolation of lactic acid bacteria (LAB) strains
HY7601 and KY1032 were isolated from kimchi, a Korean traditional dish using fermented cabbage. HY7601 and KY1032 were grown anaerobically in deMan-Rogosa- Sharpe medium at 37°C for 18 h, collected by centrifugation, and the pellets were washed with sterilized phosphate- buffered saline (PBS). The cells were stored at −20°C until use.
Preparation of aqueous extract of CR
Dried CR was purchased from Humanherb (Daegu, Korea). After washing, CR was soaked in water (dried CR : water=1:10) for 24 h at 85°C, and filtered. The filtrate was concentrated under reduced pressure in a rotary evaporator to obtain the CR extract at a yield of 6~7%. After freeze-drying, the powdered CR extract was stored at −20°C until use.
Animals, diet, and experimental design
Forty 6-week-old male C57BL/6J mice were purchased from Taconic Biosciences (Hudson, NY, USA). Two mice were housed in one cage at a constant temperature and humidity (23±2°C and 55±10%, respectively) under a 12-h light/12-h dark cycle. After 1 week for adaptation, mice were randomly divided into five groups (n=8 per group) and fed a normal diet (ND), high-fat diet (HFD), HFD with HY7601 and KY1032 [109 colony-forming units (CFU)/d], HFD with CR (CR, 2.5 mg/d), and HFD with HY7601, KY1032, and CR (LAB+CR) for 10 weeks. The probiotic and CR supplements were prepared and mixed with the diet (3 g per mouse) every week and mice were given free access to food. Food intake and body weight were measured once a week. After 10 weeks, the mice were killed under anesthesia, and blood, liver, and epididymal fat were collected. Feces were collected during the final 2 days to determine the content of fecal microbiota. The experimental procedures were approved by the Ethics Review Committee of the Korea Yakult Company Limited R&D Center, Korea (AEC-2018-00054-Y).
Histological analysis
Epididymal white adipose tissue was collected and washed with sterile PBS, fixed in 10% (v/v) formalin/PBS, and embedded in paraffin for staining with hematoxylin and eosin. The stained area was viewed under a microscope (Olympus, Tokyo, Japan), and the size of adipocytes was measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA) at a 200× magnification.
Blood analysis
The concentrations of serum leptin and adiponectin were determined using the mouse leptin immunoassay kit and mouse adiponectin immunoassay kit (R&D Systems, Minneapolis, MN, USA), respectively, according to manufacturer’s instructions. All other blood analyses were performed using TBA-40FR (Toshiba, Tokyo, Japan).
Stool sampling and extraction of total fecal DNA
Stool samples were collected from mice during the final 2 days and were stored at −80°C in a deep freezer for DNA extraction. Samples were homogenized in lysis buffer by vortexing for 1 min and the suspensions were incubated for 10 min in boiling water to lyse the cells (Ku and Lee, 2014). DNA was extracted using QIAamp DNA Stool mini kit (Qiagen, Germantown, MD, USA). The purified total stool DNA samples were quantified using a NanoDrop 2000 (Thermo Scientific, Waltham, MA, USA).
Amplification of 16S rRNA for next-generation sequencing (NGS)
The purified total stool DNA samples were diluted to a concentration of 5 ng/μL. For preparation of DNA sequencing templates, the 16S rRNA gene was amplified using polymerase chain reaction (PCR). The V3-V4 region of the 16S rRNA gene was amplified using 341 forward primer (5’-CCT ACG GGN GGC WGC AG-3’) and 805R reverse primer (5’-GAC TAC HVG GGT ATC TAA TCC-3’). The PCR mixture contained 5 μL stool DNA template (5 ng/μL), 10 μL forward primer (1 μM), 10 μL reverse primer (1 μM), and 25 μL 2× Kapa HiFi Hotstart ready mix (Kapa Biosystems, Woburn, MA, USA). The PCR products were purified using the QIAquick PCR purification kit (Qiagen) and were quantified using a Nano Drop 2000 (Thermo Scientific). After production of the 16S amplicon library, the 16S rRNA amplicons were used as template DNAs for Illumina MiSeq sequencing (Illumina, San Diego, CA, USA), according to manufacturer recommendations (McElhoe et al., 2014).
Bioinformatics analysis of NGS data
Raw data were analyzed using the QIIME package program. Analysis of raw sequence data was carried out using an Illumina Miseq and low-quality sequences were deleted during quality control. Paired-end reads were assembled using the QIIME merging script. Operational taxonomic unit (OTU) clustering of the high-quality sequences was carried out using the 16S rRNA sequences database as a reference (97% identity). The taxonomic assignment was performed using the Ribosomal Database Project (RDP) and National Center for Biotechnology Information (NCBI) database to analyze the microbiome composition of each sample. The Shannon index alpha diversity was calculated using the number of OTUs.
Statistical analysis
All data are presented as mean±standard deviation (SD). For histological and blood analysis, differences between groups were evaluated using unpaired Student’s t-tests. For bioinformatic analysis of NGS data, differences between the groups were evaluated using the Mann-Whitney U-test. All values were deemed statistically significant at P<0.05.
RESULTS
Effects of probiotics and CR extract on weight of body, liver, and epididymal fat, and size of adipocytes in diet induced obese mice
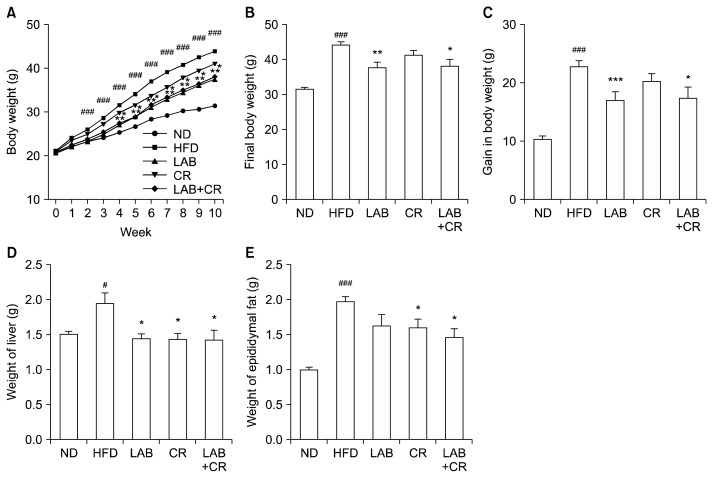
HFD supplementation for 10 weeks induced obesity in mice (Fig. 1A). Final body weight was 39% higher (P< 0.001) in the HFD fed group compared with the ND group (Fig. 1B). However, final body weight of mice fed the HFD containing LAB or LAB+CR was 14% (P<0.01) and 13% (P<0.05) lower than in the HFD fed group, respectively (Fig. 1B). Gain in body weight was 33% (P< 0.001) and 23% (P<0.05) less in the LAB and LAB+CR fed groups, respectively, compared to that of the HFD group (Fig. 1C).
Fig. 1.
Effects of probiotics and/or Cinnamomi Ramulus (CR) extract on mice fed a high-fat diet. (A) Change in body weight, (B) final body weight, (C) gain in body weight, (D) liver weight and (E) epididymal fat weight were measured. Values are presented as mean±SD. Significant differences are indicated as #P <0.05 and ###P <0.001 when compared with the normal diet group, and *P <0.05, **P <0.01, and ***P <0.001 when compared with the high-fat diet group. ND, normal diet; HFD, high-fat diet; LAB, HY7601+KY1032.
Liver weight of mice in the LAB, CR, and LAB+CR groups were also significantly lower (P<0.05) than those in the HFD group (Fig. 1D). However, weight of epididymal fat was only significantly lower than the HFD group in those fed with CR and LAB+CR (P<0.05) (Fig. 1E).
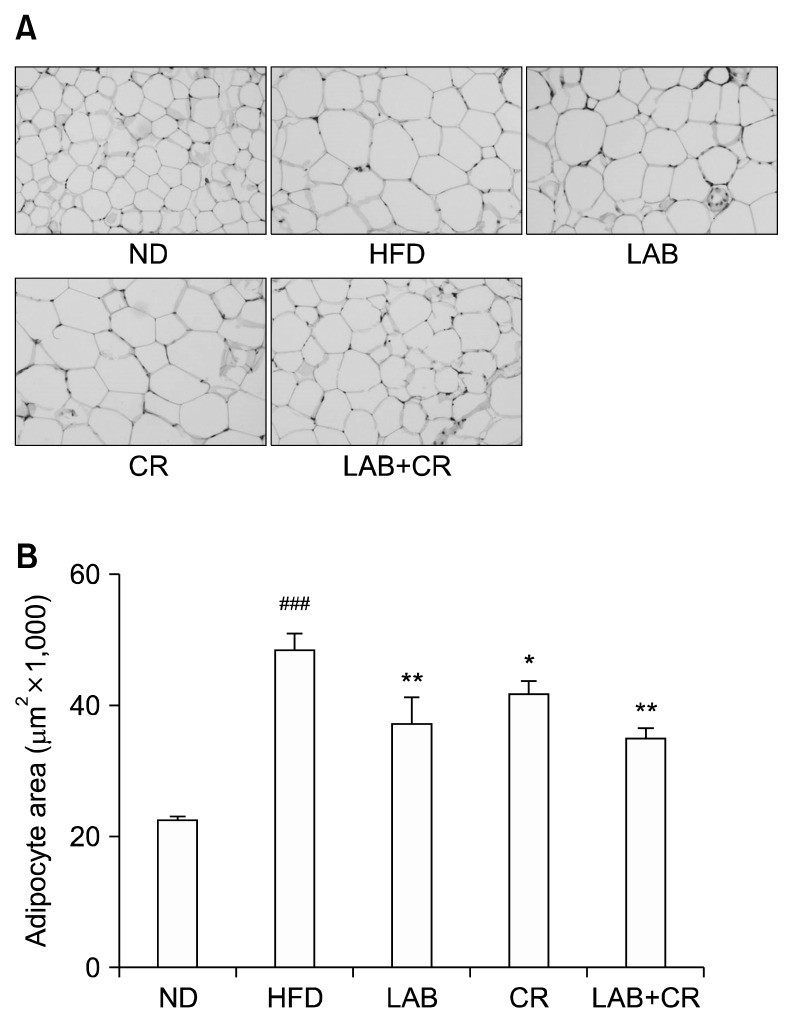
The amount of epididymal fat was measured by histological analysis. White adipose epididymal fat was markedly enlarged in mice fed the HFD compared to those in the ND group, but was reduced in mice fed LAB, CR, and LAB+CR (Fig. 2A). The histological analysis showed significant reductions in the amount of fat in the CR, LAB, and LAB+CR groups compared to the HFD group (P< 0.05, P<0.01, and P<0.01, respectively, Fig. 2B).
Fig. 2.
Effects of probiotics and/or Cinnamomi Ramulus (CR) extract on epididymal fat of mice fed a high-fat diet. (A) Morphology of epididymal fat was analyzed using a microscope, and the (B) area of epididymal fat was measured using Image J software. Values are presented as mean±SD. Significant differences are indicated as ###P <0.001 when compared with the normal diet group, and *P <0.05 and **P <0.01 when compared with the high-fat diet group. ND, normal diet; HFD, high-fat diet; LAB, HY7601+KY1032.
Effects of probiotics and CR extract on serum biochemistry, and lipid and hormone levels
The levels of liver toxicity biomarkers GOT and GPT were increased in the HFD group compared to the ND group; however, this increase was only significant for GPT (Table 1). GOT was significantly reduced in mice fed LAB and LAB+CR (P<0.01). GPT was reduced in the LAB, CR, and LAB+CR groups; however, this result was significant only in the LAB group (P<0.05). The level of lactate dehydrogenase, a marker for tissue and cell damage, was significantly increased in the HFD group (P<0.01) and decreased in mice fed LAB and LAB+CR (P<0.001 and P<0.05, respectively, Table 1).
Table 1.
Effects of probiotics and/or Cinnamomi Ramulus (CR) extract on serum biochemistry in mice fed a high-fat diet
| GOT (AST) (Unit/L) | GPT (ALT) (Unit/L) | LDH (mg/dL) | |
|---|---|---|---|
| ND | 84.00±6.93 | 39.00±11.58 | 893.80±143.94 |
| HFD | 88.40±12.03 | 116.40±48.71## | 1,145.70±69.54## |
| LAB | 55.50±6.81** | 56.50±9.29* | 800.40±61.55*** |
| CR | 90.40±10.24 | 65.20±9.11 | 1,249.70±199.32 |
| LAB+CR | 59.60±10.90** | 78.40±19.36 | 950.00±168.50* |
Values are presented as means±SD.
Significant differences are indicated as ##P<0.01 when compared with the normal diet group, and *P<0.05, **P<0.01, and ***P<0.001 when compared with the high-fat diet group.
ND, normal diet; HFD, high-fat diet; LAB, HY7601+KY1032; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic trans-aminase; LDH, lactate dehydrogenase.
Serum cholesterol levels were significantly increased in the HFD group compared with the ND group (P<0.001), but was not reduced in the LAB, CR, and LAB+CR groups (Table 2). The levels of high-density lipoprotein cholesterol were markedly increased in the HFD group compared with the ND group (P<0.001), and was reduced in the LAB, CR, and LAB+CR groups (P<0.001 for CR and LAB+CR groups, Table 2). The level of low-density lipoprotein cholesterol was significantly increased in mice fed HFD compared to the ND group (P<0.001) and was reduced in the LAB, CR, and LAB+CR groups, significant only in the LAB+CR group (P<0.05, Table 2). Serum triglyceride levels were increased 20% in the HFD group compared to the ND group (P<0.05), and were significantly reduced in mice fed LAB+CR (P<0.01, Table 2).
Table 2.
Effects of probiotics and/or Cinnamomi Ramulus (CR) extract on serum lipid profiles in mice fed a high-fat diet
| Total cholesterol (mg/dL) | HDL-cholesterol (mg/dL) | LDL-cholesterol (mg/dL) | Triglyceride (mg/dL) | |
|---|---|---|---|---|
| ND | 198.20±18.53 | 92.70±4.32 | 19.60±1.67 | 143.20±17.41 |
| HFD | 293.00±15.48### | 116.70±4.50### | 34.00±4.90### | 175.60±15.78# |
| LAB | 275.60±29.44 | 112.00±5.48 | 30.50±1.91 | 179.50±17.00 |
| CR | 295.70±28.91 | 108.70±3.27*** | 31.60±5.73 | 162.40±22.78 |
| LAB+CR | 287.30±17.47 | 100.30±3.88*** | 26.40±4.34* | 128.4±17.17** |
Values are presented as means±SD.
Significant differences are indicated as #P<0.05 and ###P<0.001 when compared with the normal diet group, and *P<0.05, **P<0.01, and ***P<0.001 when compared with the high-fat diet group.
ND, normal diet; HFD, high-fat diet; LAB, HY7601+KY1032; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
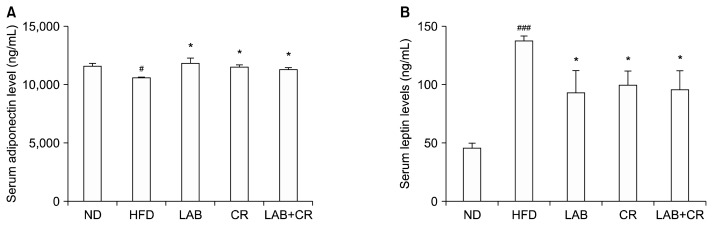
The levels of serum adiponectin were significantly decreased in mice fed the HFD compared to ND group (P< 0.05), but were increased when the HFD was supplemented with LAB, CR, and LAB+CR (P<0.05, Fig. 3A). Serum leptin levels were elevated in mice fed the HFD compared to the ND group (P<0.001), but were reduced when HFD was supplemented with LAB, CR, and LAB+ CR (P<0.05, Fig. 3B).
Fig. 3.
Effects of probiotics and/or Cinnamomi Ramulus (CR) extract on the levels of (A) adiponectin and (B) leptin in mice fed a high-fat diet. Values are presented as mean±SD. Significant differences are indicated as #P <0.05 and ###P <0.001 when compared with the normal diet group, and *P <0.05 when compared with the high-fat diet group. ND, normal diet; HFD, high-fat diet; LAB, HY7601+KY1032.
Effects of probiotics and CR extract on the diversity of gut microbiota in diet-induced obese mice
To determine changes in gut microbiota caused by dietinduced obesity, and the effect of probiotics and CR extract, fecal microbiota were analyzed by targeting the V3–V4 region of the 16S rRNA with the 341F/805R primer set using Illumina Miseq, as described above. We obtained an average of 88,569 filtered high-quality reads per sample, which were mapped to the OTUs using the NCBI and RDP database at 97% identity. We discovered an average of 5,113 OTUs per sample (Table 3).
Table 3.
Effects of probiotics and/or Cinnamomi Ramulus (CR) extract on the sequence summary in fecal samples of mouse fed a high-fat diet
| Average no. of reads | Average OTUs | |
|---|---|---|
| ND | 82,956±28,249 | 4,856±1,670 |
| HFD | 89,109±12,098 | 4,978±642 |
| LAB | 94,795±24,760 | 5,498±1,150 |
| CR | 94,532±12,213 | 5,421±493 |
| LAB+CR | 82,234±21,018 | 4,861±1,196 |
| Total | 88,569±20,233 | 5,113±1,089 |
Values are presented as means±SD.
ND, normal diet; HFD, high-fat diet; LAB, HY7601+KY1032; OTU, operational taxonomic unit.
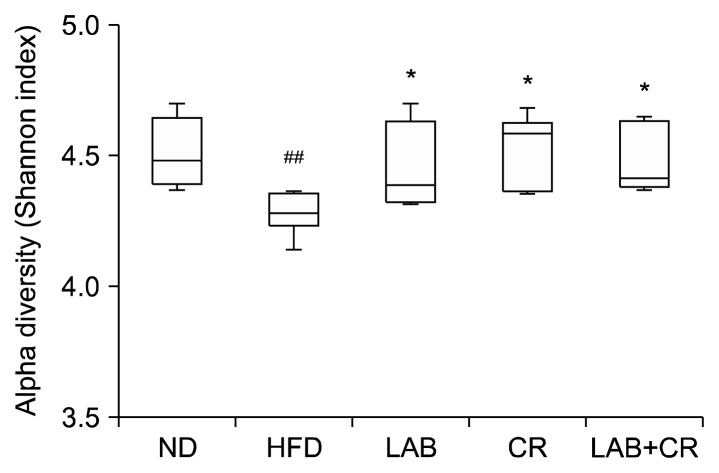
The alpha diversity of gut microbiota was measured by determining the Shannon index (Fig. 4). The Shannon index in the ND group ranged from 4.3702 to 4.6993, with an average of 4.5096; the Shannon index was significantly different in the HFD group, with an average of 4.2826 (range 4.2726 to 4.3600). The average Shannon indexes for the LAB, CR, and LAB+CR groups were 4.4600, 4.5316, and 4.4785, respectively, and were significantly higher than that of the HFD group.
Fig. 4.
Effects of probiotics and/or Cinnamomi Ramulus (CR) extract on the diversity of microbiota in mice fed a high-fat diet. Values are presented as mean±SD. Significant differences are indicated as ##P <0.01 when compared with the normal diet group and *P<0.05 when compared with the high-fat diet group. ND, normal diet; HFD, high-fat diet; LAB, HY7601+KY1032.
Effects of probiotics and CR extract on the composition of gut microbiota in diet-induced obese mice
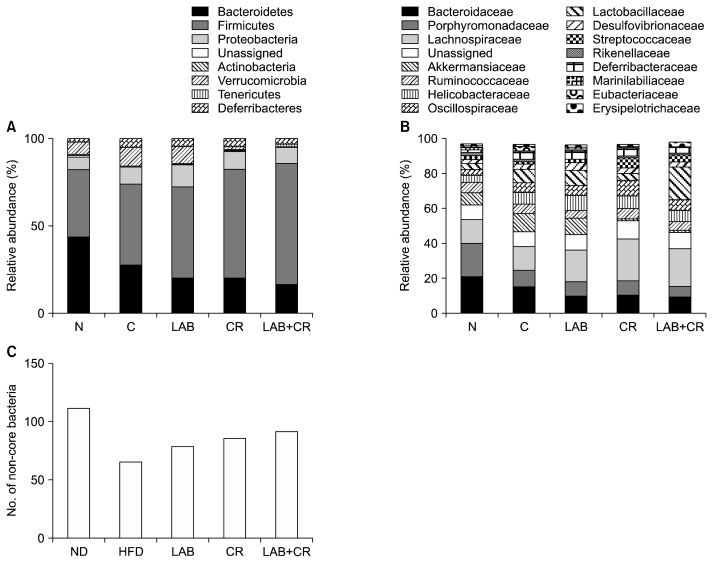
The two most abundant phyla in the gut microbiota were Firmicutes and Bacteroidetes, which comprised 70% of those in every treatment group (Fig. 5A). The abundances of Firmicutes and Bacteroidetes were increased and decreased, respectively, in the HFD group compared to the ND group. The ratios of Firmicutes and Bacteroidetes abundances were further increased in the LAB, CR, and LAB+CR groups compared to the ND group. In addition, the abundance of Deferribacteres was significantly increased in the HFD group compared to the ND group, but was decreased in the LAB and LAB+CR groups compared to the HFD group (changes not significant). The reads of the phylum Tenericutes were also significantly decreased in the LAB group compared to the HFD group (Fig. 5A).
Fig. 5.
Effects of probiotics and/or Cinnamomi Ramulus (CR) extract on the composition of gut microbiota at (A) phylum and (B) family levels, and (C) the number of genera in the gut microbiota in mice fed a high-fat diet. ND, normal diet; HFD, high-fat diet; LAB, HY7601+KY1032.
We further analyzed differences in the composition of gut microbiota at the family level (Fig. 5B). We found that all treatment groups had an increased percentage of Lactobacillaceae and Lachnospiraceae compared to the ND group, with the largest increase observed in the LAB+ CR group.
When analyzed at the genus level, the total number of genera in the five groups was 490, with only 139 genera commonly shared in all five groups (which included the core bacteria). The ND group comprised of 139 core and 111 non-core bacteria, and had the largest diversity, whereas the HFD group comprised of 139 core and 65 non-core bacteria. The LAB, CR, and LAB+CR groups had 78, 85, and 91 non-core bacteria, respectively, higher in all cases than the HFD group (Fig. 5C).
DISCUSSION
Obesity is a major health issue. Obesity has become a global healthcare burden as it is closely related to metabolic diseases, including type 2 diabetes, cardiovascular disease, and several types of cancer (Bray, 2000; Leonhardt et al., 1999). There are various medications for inducing weight loss, however since all have side effects, safe and effective treatment strategies are warranted. In the present study, we investigated the anti-obesity effects of different combinations of HY7601 and KY1032 with CR extract, and determined the how they modify intestinal microbiota in high-fat diet-fed mice.
Mice fed the HFD diet for 10 weeks gained 39% more weight compared to the ND-fed mice. The weight gain correlated to increases in liver and epididymal fat weight, which were increased 98% and 29% more than in ND-fed mice, respectively. However, supplementation with probiotics, CR, or both alleviated the gain in body, liver, and epididymal fat weight. The area of adipose tissue in epididymal fat was also reduced, especially in the group fed probiotics and CR, which showed a synergistic effect.
Diet-induced obesity models are prone to increases in levels of plasma cholesterol and triglycerides, which are commonly associated with dyslipidemia (Do et al., 2011). Our study showed that both probiotics and CR reduced blood cholesterol and triglyceride levels. Previous studies have reported that probiotics lower plasma cholesterol, likely through a mechanism where probiotics promote fecal excretion (Xie et al., 2011; Paik et al., 2005). Alternatively, cholesterol can be metabolized by resident intestinal microbiota (Veiga et al., 2005). Although the major factors involved in the cholesterol-lowering activity of microbes have not yet been elucidated, our data show that for HY7601 and KY1032 this activity is promoted by the presence of CR.
Adiponectin is a hormone produced by adipocytes that acts as an anti-diabetic, anti-atherogenic, and anti-inflammatory adipocytokine (Masuyama et al., 2015). Decreases in the level of adiponectin in circulation are associated with obesity, insulin resistance, and type 2 diabetes (Stefan et al., 2002; Lihn et al., 2005). Leptin is a hormone exclusively secreted by adipocytes, and is involved in regulating the balance between food intake and energy expenditure (Cho et al., 2008). Leptin is an indicator of obesity as its secretion depends on the proportion of stored triglycerides, and its concentration in circulation correlates obesity (Maffei et al., 1995; Havel, 2000). In this study, treatment with probiotics, CR, or both increased the concentration of adiponectin and decreased the concentration of leptin in the blood.
Recent metagenomics data suggests that a low-diversity in the fecal microbiome is related to adiposity and metabolic disturbances, due to a decreased capability to produce butyrate (Daniel et al., 2014). In agreement with the results of this study, the alpha diversity in HFD-fed mice was significantly decreased, whereas supplementation of LAB, CR, or both did not induce significant changes compared to that of the ND-fed mice.
Firmicutes and Bacteroidetes are the most abundant members of the intestinal microbiota. The ratio of these bacterial groups can change due to different factors, including obesity (Zhang et al., 2012; Ley et al., 2005). Our study showed an increase in the F/B ratio in HFD-fed mice compared to the ND-fed mice, and was increased further in the LAB, CR, and LAB+CR groups. This might be due to the increase in the abundance of Lachnospiraceae, which belongs to Firmicutes. Lachnospiraceae is known to produce butyrate, which prevents colon cancer and fat accumulation (Meehan and Beiko, 2014; Duncan et al., 2008). Deferribacteres, another phylum that is present in the mouse gut, contains Mucispirillum, the abundance of which increases in HFD-fed mice (Ravussin et al., 2012). The HFD group had an increased abundance of Deferribacteres compared with the ND-fed group, whereas the abundance was low in the LAB+CR group.
At the family level, we discovered an increase in the abundance of Lactobacillaceae in mice fed LAB+CR. Both the LAB and LAB+CR groups were supplemented with the same CFU of LAB; however, the LAB+CR group had a significantly higher ratio of Lactobacillaceae, which leads to the conclusion that CR might help HY7601 and KY1032 to settle in the gut.
Short chain fatty acids are the primary end-products produced by the gut microbiota, and act as metabolic substrates regulating the cellular metabolism in the host (Morrison and Preston, 2016). Acetatifactor is a genus that produces acetate and butyrate in the gut (Pfeiffer et al., 2012). Our data show that the ratio of Acetatifactor was not significantly altered in the HFD group compared to the ND group, but was significantly increased in the LAB, CR, and LAB+CR groups (data not shown).
In the present study, we showed that the gain in body weight and several other symptoms of obesity were alleviated, and the gut microbiota was altered in diet-induced obese mice treated with a mixture of HY7601, KY1032, and CR. These findings suggest that the synergic effect of HY7601, KY1032, and CR, which modulates gut microbiota, may represent a natural alternative for alleviation of obesity.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- Ahn HY, Kim M, Chae JS, Ahn YT, Sim JH, Choi ID, et al. Supplementation with two probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032, reduces fasting triglycerides and enhances apolipoprotein A-V levels in non-diabetic subjects with hypertriglyceridemia. Atherosclerosis. 2015;241:649–656. doi: 10.1016/j.atherosclerosis.2015.06.030. [DOI] [PubMed] [Google Scholar]
- Bray GA. A concise review on the therapeutics of obesity. Nutrition. 2000;16:953–960. doi: 10.1016/S0899-9007(00)00424-X. [DOI] [PubMed] [Google Scholar]
- Cho EJ, Rahman MA, Kim SW, Baek YM, Hwang HJ, Oh JY, et al. Chitosan oligosaccharides inhibit adipogenesis in 3T3-L1 adipocytes. J Microbiol Biotechnol. 2008;18:80–87. [PubMed] [Google Scholar]
- Daniel H, Gholami AM, Berry D, Desmarchelier C, Hahne H, Loh G, et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014;8:295–308. doi: 10.1038/ismej.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBaise JK, Zhang H, Crowell MD, Krajmalnik-Brown R, Decker GA, Rittmann BE. Gut microbiota and its possible relationship with obesity. Mayo Clin Proc. 2008;83:460–469. doi: 10.4065/83.4.460. [DOI] [PubMed] [Google Scholar]
- Do GM, Oh HY, Kwon EY, Cho YY, Shin SK, Park HJ, et al. Long-term adaptation of global transcription and metabolism in the liver of high-fat diet-fed C57BL/6J mice. Mol Nutr Food Res. 2011;55:S173–S185. doi: 10.1002/mnfr.201100064. [DOI] [PubMed] [Google Scholar]
- Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes. 2008;32:1720–1724. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- Gan CC, Ni TW, Yu Y, Qin N, Chen Y, Jin MN, et al. Flavonoid derivative (Fla-CN) inhibited adipocyte differentiation via activating AMPK and up-regulating microRNA-27 in 3T3-L1 cells. Eur J Pharmacol. 2017;797:45–52. doi: 10.1016/j.ejphar.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Han Y, Jung HW, Bae HS, Kang JS, Park YK. The extract of Cinnamomum cassia twigs inhibits adipocyte differentiation via activation of the insulin signaling pathway in 3T3-L1 preadipocytes. Pharm Biol. 2013;51:961–967. doi: 10.3109/13880209.2013.772211. [DOI] [PubMed] [Google Scholar]
- Havel PJ. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proc Nutr Soc. 2000;59:359–3571. doi: 10.1017/S0029665100000410. [DOI] [PubMed] [Google Scholar]
- Heck AM, Yanovski JA, Calis KA. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy. 2000;20:270–279. doi: 10.1592/phco.20.4.270.34882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeung WH, Shim JJ, Woo SW, Sim JH, Lee JL. Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 cell extracts inhibit adipogenesis in 3T3-L1 and HepG2 cells. J Med Food. 2018;21:876–886. doi: 10.1089/jmf.2017.4157. [DOI] [PubMed] [Google Scholar]
- Jung J, Lee JH, Bae KH, Jeong CS. Anti-gastric actions of eugenol and cinnamic acid isolated from Cinnamomi Ramulus. Yakugaku Zasshi. 2011;131:1103–1110. doi: 10.1248/yakushi.131.1103. [DOI] [PubMed] [Google Scholar]
- Kang YH, Shin HM. Cinnamomi ramulus ethanol extract exerts vasorelaxation through inhibition of Ca2+ influx and Ca2+ release in rat aorta. Evid Based Complement Alternat Med. 2012;2012 doi: 10.1155/2012/513068. 513068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku HJ, Lee JH. Development of a novel long-range 16S rRNA universal primer set for metagenomic analysis of gastrointestinal microbiota in newborn infants. J Microbiol Biotechnol. 2014;24:812–822. doi: 10.4014/jmb.1403.03032. [DOI] [PubMed] [Google Scholar]
- Leonhardt M, Hrupka B, Langhans W. New approaches in the pharmacological treatment of obesity. Eur J Nutr. 1999;38:1–13. doi: 10.1007/s003940050040. [DOI] [PubMed] [Google Scholar]
- Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- Masuyama H, Mitsui T, Nobumoto E, Hiramatsu Y. The effects of high-fat diet exposure in utero on the obesogenic and diabetogenic traits through epigenetic changes in adiponectin and leptin gene expression for multiple generations in female mice. Endocrinology. 2015;156:2482–2491. doi: 10.1210/en.2014-2020. [DOI] [PubMed] [Google Scholar]
- McElhoe JA, Holland MM, Makova KD, Su MS, Paul IM, Baker CH, et al. Development and assessment of an optimized next-generation DNA sequencing approach for the mtgenome using the Illumina MiSeq. Forensic Sci Int Genet. 2014;13:20–29. doi: 10.1016/j.fsigen.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan CJ, Beiko RG. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol Evol. 2014;6:703–713. doi: 10.1093/gbe/evu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik HD, Park JS, Park E. Effects of Bacillus polyfermenticus SCD on lipid and antioxidant metabolisms in rats fed a high-fat and high-cholesterol diet. Biol Pharm Bull. 2005;28:1270–1274. doi: 10.1248/bpb.28.1270. [DOI] [PubMed] [Google Scholar]
- Park DY, Ahn YT, Park SH, Huh CS, Yoo SR, Yu R, et al. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One. 2013;8:e59470. doi: 10.1371/journal.pone.0059470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer N, Desmarchelier C, Blaut M, Daniel H, Haller D, Clavel T. Acetatifactor muris gen. nov., sp. nov., a novel bacterium isolated from the intestine of an obese mouse. Arch Microbiol. 2012;194:901–907. doi: 10.1007/s00203-012-0822-1. [DOI] [PubMed] [Google Scholar]
- Rahman HA, Sahib NG, Saari N, Abas F, Ismail A, Mumtaz MW, et al. Anti-obesity effect of ethanolic extract from Cosmos caudatus Kunth leaf in lean rats fed a high fat diet. BMC Complement Altern Med. 2017;17:122. doi: 10.1186/s12906-017-1640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin Y, Koren O, Spor A, LeDuc C, Gutman R, Stombaugh J, et al. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity. 2012;20:738–747. doi: 10.1038/oby.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan N, Stumvoll M. Adiponectin – its role in metabolism and beyond. Horm Metab Res. 2002;34:469–474. doi: 10.1055/s-2002-34785. [DOI] [PubMed] [Google Scholar]
- Suppapitiporn S, Kanpaksi N, Suppapitiporn S. The effect of Cinnamon cassia powder in type 2 diabetes mellitus. J Med Assoc Thai. 2006;89:S200–S205. [PubMed] [Google Scholar]
- Veiga P, Juste C, Lepercq P, Saunier K, Béguet F, Gérard P. Correlation between faecal microbial community structure and cholesterol- to-coprostanol conversion in the human gut. FEMS Microbiol Lett. 2005;242:81–86. doi: 10.1016/j.femsle.2004.10.042. [DOI] [PubMed] [Google Scholar]
- Xie N, Cui Y, Yin YN, Zhao X, Yang JW, Wang ZG, et al. Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. BMC Complement Altern Med. 2011;11:53. doi: 10.1186/1472-6882-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SR, Kim YJ, Park DY, Jung UJ, Jeon SM, Ahn YT, et al. Probiotics L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-induced obesity. Obesity. 2013;21:2571–2578. doi: 10.1002/oby.20428. [DOI] [PubMed] [Google Scholar]
- Yun JW. Possible anti-obesity therapeutics from nature – a review. Phytochemistry. 2010;71:1625–1641. doi: 10.1016/j.phytochem.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhang M, Pang X, Zhao Y, Wang L, Zhao L. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 2012;6:1848–1857. doi: 10.1038/ismej.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]