Abstract
Cudrania tricuspidata has been used in East Asia as a folk medicine for symptoms such as inflammation, allergy, and gastritis. Administration of C. tricuspidata extract to pylori-ligated rat stomachs reduces gastric acid secretion and alleviates esophagus damage caused by gastric reflux. Therefore, in this study we aimed to investigate whether C. tricuspidata extracts inhibit reflux esophagitis by blocking H2 histamine receptor (H2R). Dimaprit, a H2R specific agonist, induced intracellular cyclic adenosine monophosphate (cAMP) production in U937 cells. Pretreatment with C. tricuspidata extracts significantly blocked dimaprit-induced cAMP production in a concentration-dependent manner. To extracted C. tricuspidata with different ethanol concentrations to determine the optimum method. We found that the 70% ethanol extract showed the most potent H2R antagonistic effect against dimaprit-induced cAMP production. However, water extract did not show any H2R blocking effect. These findings suggest that C. tricuspidata extracted using ethanol specifically inhibits gastric acid secretion and reduces esophageal injury by blocking H2R in a competitive manner. Therefore, C. tricuspidata extracts may be used in food or medicine to prevent H2R-related diseases, such as gastric hyperacidity and reflux esophagitis.
Keywords: Cudrania tricuspidata, H2 histamine receptor, reflux esophagitis, gastric juice secretion, gastric hyperacidity
INTRODUCTION
Gastroesophageal reflux disease (GERD), which includes reflux esophagitis, is a disease during which stomach contents (mainly acid and pepsin) are refluxed into the esophagus, resulting in various clinical symptoms and mucosal changes. Reflux esophagitis is a chronic condition characterized by severe lesions caused by prolonged exposure to acid. In general, strong acidic gastric juice stimulates the esophagus to induce refluxing accompanied by a burning sensation from the chest to the throat, leading to chest tightness, breathing difficulty, and, in severe cases, heartburn. Frequent coughing and reflux secretions lead to bitter tastes in the mouth from which aspiration pneumonia may develop as a complication (Rieder et al., 2010; Moore et al., 2016).
The main cause of reflux esophagitis is eating habits. Intakes too high in fatty, fried, or spicy food can increase gastric acid secretion, while overeating and rapid food intake increase gastrointestinal pressure which makes it difficult to neutralize stomach acid. Another cause of reflux esophagitis is the habit of lying down straight after meals. Lying down after overeating is often linked to reflux esophagitis. Moreover, cigarettes, caffeine, soft drinks, chocolate, peppermint, and orange juice can weaken the squeezing ability of the sphincter, causing reflux esophagitis (Eslick and Talley, 2009; Hsu et al., 2013; Henry, 2014). The most commonly used drugs for treatment of reflux esophagitis are proton pump inhibitors and histamine H2 receptor (H2R) blockers. These drugs lower the acidity of gastric juice and reduce irritations when gastric reflux occurs (Vakil et al., 2006; Abdul-Hussein et al., 2015).
The genes encoding four histamine receptor subtypes (H1, H2, H3, and H4) have been cloned, and the receptors and their downstream signaling pathways have been pharmacologically characterized (Repka-Ramirez, 2003). H2R is a G protein-coupled receptor that couples with adenylyl cyclase, which produces the intracellular second messenger cyclic adenosine monophosphate (cAMP) (Klinker et al., 1996a; Klinker et al., 1996b). In general, H2R is present in gastric parietal cells and regulates secretion signals in the stomach. Activation of H2R in gastric parietal cells increases production of cAMP and induces release of hydrogen ions (H+) in the gastric juice by proton pump, leading to increased gastric juice acidity. Therefore, H2R blockers may be useful for treating diseases that involve gastric acid hypersecretion, such as gastric ulcers and reflux esophagitis (Kowalsky et al., 1991; Onodera et al., 1999; Weiser et al., 1983).
Cudrania tricuspidata is a deciduous tree that has been used in traditional medicine to treat eczema, mumps, pulmonary tuberculosis, allergy, and acute arthritis (Xin et al., 2017). It has been reported that different parts of the tree (fruit, root, stem, and leaf) may exert beneficial antiinflammatory, anti-obesity, and antioxidant effects (Park et al., 2006; Lee et al., 2006; Kim et al., 2016; Jo et al., 2017). Although there is increasing evidence for the different physiological activities induced by C. tricuspidata, the molecular mechanisms of action of C. tricuspidata on GERD and reflux esophagitis have not yet been elucidated.
In the present study, we used the U937 monocyte cell line and pylorus-ligated rat models to evaluate the potential activity of C. tricuspidata as an H2R antagonist and reflux esophagitis agent. The aim of this study is to provide evidence that ethanol extracts of C. tricuspidata leaves negatively regulate H2R activity and apparently act as specific and competitive H2R antagonists in terms of the secretion blockage of gastric acid similar to that of ranitidine.
MATERIALS AND METHODS
Preparation of C. tricuspidata extract
Dried C. tricuspidata leaves were pulverized to an appropriate size and placed in an extraction vessel. Ethanol (0% to 70%, corresponding to 10 times the weight of the leaves) was added to the extraction vessel, refluxed, and stirred at 50°C for 6 h. C. tricuspidata extracts were adsorbed and filtered by perlite to remove insoluble impurities. The filtered juice extract was concentrated using a rotary vacuum evaporator (EYELA, Tokyo, Japan), lyophilized, pulverized, and powdered. The dried materials were resuspended in 0.5% methyl cellulose (MC) for animal experiments and in dimethyl sulfoxide (DMSO) for cellular experiments.
Animals
Six weeks old male Sprague-Dawley rats were maintained at a temperature of 23±3°C, a relative humidity of 55± 15%, a ventilation frequency of 10~20 times/h, and with 12 h of light (8:00 am to 8:00 pm off). Temperature and relative humidity were measured every hour using a computer system, and the frequency of ventilation and illumination was measured periodically. There were no abnormalities that could affect the test results during the experiments. All studies were approved by the Institutional Animal Care and Use Committee of Gyeonggi Bio Research Center (approval no. 2016-10-0011, 2017-05-0005).
Gastric secretions and gastric acidity measurements
Animals were fasted for 24 h before administration of test substances. Test substances (C. tricuspidata 70% ethanol extract or ranitidine) were suspended in 0.5% MC before administration. In the vehicle group, only 5% MC was administered. One hour after oral administration of test substances, animals were anesthetized with isoflurane, and the pylorus was ligated. After 8 h of pyloric ligation, animals were sacrificed with CO2 gas and gastric juice was collected from the stomach using a 10-mL syringe. The gastric juice was centrifuged at 3,000 rpm for 10 min, the supernatant removed, and the gastric fluid amount (mL), pH, and acidity were measured. To determine gastric acidity, 1 mL of centrifuged gastric juice was dispensed into a tube and titrated to pH 7.0 with 0.1 N NaOH; the amount of 0.1 N NaOH used in the titration was measured. Total acidity was calculated using the following formula:
Induction of reflux esophagitis and esophageal lesion measurement
All animals were fasted for 36 h before test substances were administered. One hour after oral administration of test substance (C. tricuspidata 70% ethanol extract or ranitidine), animals were anesthetized with tiletamine/ zolazepam (10 mg/kg; Zoletil 50, Virbac, Carros, France) and 2% xylazine hydrochloride (2 mg/kg, Rumpun, Byer Co., Seoul, Korea). The abdomen of the anesthetized rats was shaved and disinfected with povidone, followed by incision along the midline at 4 to 5 cm. The exposed pylorus and the limiting ridge were ligated with silk (3-0, B. Braun Surgical S.A., Barcelona, Spain). The peritoneum was closed with 3/0 absorbable suture (3/0 Surgisorb, Samyang, Seoul, Korea), and the skin was ligated with silk (3-0, B. Braun Surgical S.A.). Eight hours following the operations, animals were sacrificed with CO2 gas, and the stomachs and esophagi were collected. Stomachs and esophagi were cut longitudinally using surgical scissors, and the blood was washed with phosphate buffered saline. The dissected stomachs and esophagi were spread on clean paper and photographed with a digital camera (Coolpix P5100, Nikon, Tokyo, Japan) under 200 to 300 lux illumination at the designated site. The distance between the camera and the specimen was measured with object markers and scale bars (mm unit) at the time of photographing. The lesion area of injured esophagus mucosa was measured using Image J (Wayne Rasband; National Institutes of Health, Bethesada, MD, USA).
Cell culture
The U937 cell line (American Type Culture Collection, Rockville, MD, USA) was suspended in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% (v/v) heat-inactivated fetal calf serum and 1% calcium phosphate buffer at 37°C.
Measurement of intra-cellular cAMP
U937 cells were cultured in RPMI-1640 medium, centrifuged at 200 g, and suspended in RPMI-1640 medium containing 10 μM RO-20-1724 (Sigma, St. Louis, MO, USA). Cell suspensions were pretreated with C. tricuspidata extracts or 10 μM of ranitidine hydrochloride (Sigma) for 5 min and then treated with 10 μM of H2R specific agonist dimaprit (Sigma) for 20 min. After 20 min of dimaprit treatment, U937 cells recovered by centrifugation (1,800 g) were treated with cAMP assay cell lysis buffer (R&D Systems, Minneapolis, MN, USA) and immediately frozen. The frozen cells were disrupted by repeatedly freezing and thawing three times, centrifuged at 2,000 g for 15 min, and cAMP production was measured in the supernatant. The cAMP assay was performed using a cAMP assay kit from R&D Systems.
Statistical analysis
Experimental results were expressed as mean±standard error (SE) and analysed using SPSS (version 20, IBM SPSS Corp., Armonk, NY, USA). Levene’s test was performed to compare the variance homogeneity for all data. Oneway ANOVA with Tukey’s post hoc mean separation tests was used to analyze significance when the variance was homogeneous.
RESULTS
Effect of C. tricuspidata on gastric acid secretion
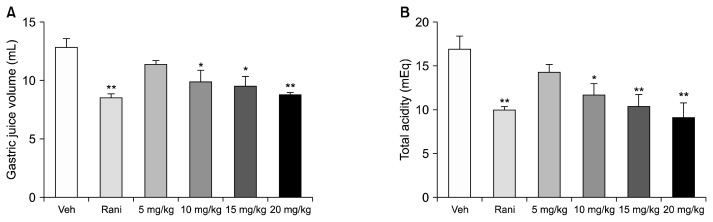
The pylori-ligated rat model (Satyanarayana et al., 1989) was used to investigate the effects of C. tricuspidata extracts on total gastric volume and total acidity of gastric juice. Analysis of gastric contents showed that C. tricuspidata extracts decreased the amount and total acidity of gastric juices in a concentration-dependent manner. Specifically, 20 mg/kg of C. tricuspidata extract showed an inhibitory effect on gastric acid secretion almost comparable to the H2R inhibitor ranitidine (Fig. 1). The C. tricuspidata ethanol extract decreased gastric acid secretion following pylorus ligature, indicating that this may be the mechanism by which C. tricuspidata extracts protect the esophagus mucosa.
Fig. 1.
Effect of Cudrania tricuspidata extract on gastric acid secretion. (A) Total gastric juice secretion after pylorus ligature and (B) total acidity of gastric contents from pylorus-ligated rats. Gastric juice was collected immediately after sacrificing the rats, and the total volume and total acidity of gastric secretions were measured. The pylorus-ligated rats were treated with the indicated dose of C. tricuspidata extract. Veh, 0.5% methyl cellulose; Rani, 7.7 mg/kg of ranitidine; 5 mg/kg, 5 mg/kg of C. tricuspidata extract; 10 mg/kg, 10 mg/kg of C. tricuspidata extract; 15 mg/kg, 15 mg/kg of C. tricuspidata extract; 20 mg/kg, 20 mg/kg of C. tricuspidata extract. Values are mean±SE. Significantly different from Veh group at *P <0.05 and **P <0.01.
Effect of C. tricuspidata on reflux esophagitis
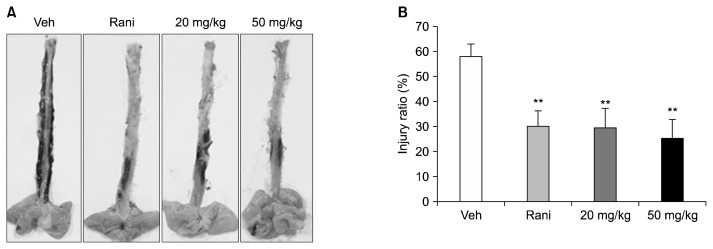
In the vehicle group, most of the esophageal mucosa were damaged by stomach acid, resulting in severe redness. In contrast, rats treated with ranitidine and C. tricuspidata extracts showed a decrease in redness due to gastric acid secretion (Fig. 2A). The esophageal injury ratio of the vehicle group was 57.72±5.16%. C. tricuspidata extracts at concentrations of 20 and 50 mg/kg attenuated the esophageal injury ratio to 29.41±7.84% and 25.18±7.57%, respectively. Ranitidine at 7.7 mg/kg also attenuated the lesion score to 29.90±6.3% (Fig. 2B, Table 1). The C. tricuspidata extract-treated group did not show dose-dependent inhibition of esophageal mucosal damage, but the mean level of damage was comparable to that of the ranitidine-treated group. The high-dose C. tricuspidata extract-treated group (50 mg/kg) showed the greatest reduction of esophageal mucosal damage.
Fig. 2.
Effect of Cudrania tricuspidata extract on reflux esophagitis. (A) Esophageal lesions and (B) the area ratio of esophageal injury. The esophagus was collected immediately after rats were sacrificed and was cut in the longitudinal direction from the gastroesophageal junction to the pharynx. The dissected esophagus was laid on paper, and photographic images were captured with an optical digital camera. The pylorus- and forestomach-ligated rats were treated with the indicated dose of C. tricuspidata extract. Veh, 0.5% methyl cellulose; Rani, 7.7 mg/kg of ranitidine; 20 mg/kg, 20 mg/kg of C. tricuspidata extract; 50 mg/kg, 50 mg/kg of C. tricuspidata extract. Values are mean±SE. Significantly different from Veh group at **P <0.01.
Table 1.
Gross esophageal damage in rats with reflux esophagitis
| Groups | Total area (mm2) | Lesion area (mm2) | Injury ratio (%) |
|---|---|---|---|
| G1 | 448.18±21.71 | 261.75±30.22 | 57.72±5.16 |
| G2 | 434.04±26.08 | 136.05±33.67 | 29.90±6.30** |
| G3 | 398.89±20.90 | 116.54±31.23 | 29.41±7.84** |
| G4 | 408.04±12.68 | 106.60±32.60 | 25.18±7.57** |
Data were expressed as mean±SE.
The results were statistically analyzed by one-way ANOVA.
Significantly different from G1 at P <0.01.
G1, vehicle (0.5 % MC, 10 mL/kg, n=8); G2, ranitidine hydrochloride (7.7 mg/kg, n=8); G3, C. tricuspidata extract (20 mg/kg, n=8); G4, C. tricuspidata extract (50 mg/kg, n=8).
C. tricuspidata inhibits H2R-mediated cAMP production in U937 cells
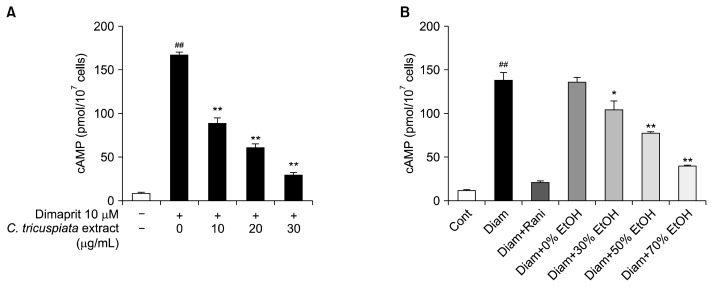
H2R is a major target of anti-ulcer drugs (Kowalsky et al., 1991). Inhibitory effects of H2R blockers on gastric acid secretion have been demonstrated in many animal model systems (Konturek et al., 1980; Ohsawa et al., 2002; Kim et al., 2005). H2R is expressed in immune cells, such as the U937 cell line, which is widely used as a model system for H2R activation and GI tract cells (Kim et al., 2005; Jutel et al., 2001; Delgado et al., 2002). Therefore, we investigated whether C. tricuspidata extracts inhibited cAMP production by dimaprit, a selective H2R agonist, in U937 cells. Treatment of U937 cells with dimaprit resulted in a significant increase in cAMP, which was inhibited by C. tricuspidata extract in a dose-dependent manner (Fig. 3A).
Fig. 3.
Inhibition of H2 histamine receptor (H2R)-mediated cyclic adenosine monophosphate (cAMP) production by Cudrania tricuspidata extract in U937 cells. (A) C. tricuspidata ethanol extracts inhibit cAMP production in a dose-dependent manner. (B) H2R inhibitory activity of C. tricuspidata extracted with various concentrations of ethanol. U937 cells were pretreated with 10 μM of ranitidine or C. tricuspidata extracts for 5 min. Next, cells were stimulated with 10 μM of dimaprit. The control group was treated with the same amount of DMSO instead of ranitidine or C. tricuspidata extracts. Values are mean±SE. ##P <0.01 indicates a significant difference between the control group and the Dimaprit group. A significant difference between the Dimaprit group and the C. tricuspidata extract groups at *P <0.05 and **P <0.01.
In the above animal experiments, we found that the C. tricuspidata 70% ethanol extract effectively inhibited gastric acid secretion and reflux esophagitis. Therefore, we investigated the effects of various concentrations of C. tricuspidata ethanol extractions on H2R inhibition. Higher ethanol concentration induced greater H2R inhibition. The 0% ethanol extract (hot water extract) did not affect H2R inhibition (Fig. 3B). Ranitidine, a H2R-specific antagonist, almost completely inhibited cAMP production by H2R at a concentration of 10 μM (Fig. 3B). When ethanol was used as the solvent at concentrations over 70%, only a very small amount of extract was obtained.
There was no cellular damage in U937 cells treated with ranitidine or C. tricuspidata extracts (data not shown), suggesting that the inhibitory effects of H2R by C. tricuspidata extracts was not due to inducing cell death. We also measured the H2R inhibitory effect of C. tricuspidata fruit and stem extracts; the fruit extract did not inhibit H2R, and the stem extract only showed a small inhibitory effect (data not shown).
DISCUSSION
C. tricuspidata has long been used for treatment of gastrointestinal diseases in folk medicine. Although many studies have reported the effects of C. tricuspidata on allergy, inflammation, diabetes, and obesity (Kim et al., 2016; Jo et al., 2017; Lee et al., 2012; Kim et al., 2015; Jo et al., 2014; You et al., 2017), the mechanism of action is unclear for gastrointestinal diseases such as gastritis and reflux esophagitis. In this study, we suggest that C. tricuspidata ethanol extracts may block H2R and prevent gastric hyperacidity and reflux esophagitis.
A single oral administration of C. tricuspidata ethanol extract reduced gastric acid secretion in pyloric-ligated animals, similar to the effects of ranitidine (Fig. 1), and effectively suppressed esophageal damage in the reflux esophagitis model (Fig. 2). These results suggest that C. tricuspidata ethanol extracts inhibit signals related to gastric acid secretion. Thus, we determined whether C. tricuspidata inhibits H2R-mediated cAMP production in U937 cells. We found that the ethanol extract of C. tricuspidata inhibited H2R activity in a concentration-dependent manner (Fig. 3A). Inhibition of cAMP signaling by C. tricuspidata ethanol extracts was due to blockade of histamine binding to H2R, rather than accelerating degradation, based on inhibition observed in the presence of the phosphodiesterase inhibitor RO-20-1724. In addition, when C. tricuspidata was extracted with high concentration of ethanol, higher H2R inhibitory activity was observed compared with extraction with low concentrations of ethanol or water. These results suggest that the active component of C. tricuspidata that inhibits H2R is a hydrophobic, and not hydrophilic material. Therefore, we are conducting a follow-up study to identify a single component that specifically inhibits H2R in C. tricuspidata by fractionating the C. tricuspidata extract with hydrophobic solvents such as ethyl acetate.
Antagonism of H2R has been the cornerstone of pharmacological treatment of gastrointestinal tract acid disorders, such as gastric hyperplasia and gastritis (Kowalsky et al., 1991). Selective H2R inhibition and gastric acid secretion by C. tricuspidata indicates that C. tricuspidata extracts or its active ingredients may be a promising candidate for the treatment of gastric ulcers and other H2R-related diseases. Therefore, further studies are needed to demonstrate the effect of C. tricuspidata on gastrointestinal dysfunction, such as hypergastric acidity and reflux esophagitis, in humans, and to identify individual C. tricuspidata components that inhibit H2R.
In conclusion, our data show that C. tricuspidata extract can inhibit H2R and may effectively prevent diseases such as gastric hyperacidity and reflux esophagitis associated with H2R-mediated gastric secretion.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- Abdul-Hussein M, Freeman J, Castell D. Concomitant administration of a histamine2 receptor antagonist and proton pump inhibitor enhances gastric acid suppression. Pharmacotherapy. 2015;35:1124–1129. doi: 10.1002/phar.1665. [DOI] [PubMed] [Google Scholar]
- Delgado M, Fernández-Alfonso MS, Fuentes A. Effect of adrenaline and glucocorticoids on monocyte cAMP-specific phosphodiesterase (PDE4) in a monocytic cell line. Arch Dermatol Res. 2002;294:190–197. doi: 10.1007/s00403-002-0313-3. [DOI] [PubMed] [Google Scholar]
- Eslick GD, Talley NJ. Gastroesophageal reflux disease (GERD): risk factors, and impact on quality of life-a population-based study. J Clin Gastroenterol. 2009;43:111–117. doi: 10.1097/MCG.0b013e31815ea27b. [DOI] [PubMed] [Google Scholar]
- Henry MA. Diagnosis and management of gastroesophageal reflux disease. Arq Bras Cir Dig. 2014;27:210–215. doi: 10.1590/S0102-67202014000300013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PI, Kim N, Goh KL, Wu DC. Diagnosis and management of gastroesophageal reflux disease. Gastroenterol Res Pract. 2013;2013 doi: 10.1155/2013/709620. 709620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Kim SB, Liu Q, Hwang BY, Lee MK. Prenylated xanthones from the roots of Cudrania tricuspidata as inhibitors of lipopolysaccharide-stimulated nitric oxide production. Arch Pharm. 2017;350:e1600263. doi: 10.1002/ardp.201600263. [DOI] [PubMed] [Google Scholar]
- Jo YH, Shin B, Liu Q, Lee KY, Oh DC, Hwang BY, et al. Antiproliferative prenylated xanthones and benzophenones from the roots of Cudrania tricuspidata in HSC-T6 cells. J Nat Prod. 2014;77:2361–2366. doi: 10.1021/np5002797. [DOI] [PubMed] [Google Scholar]
- Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OA, Malolepszy J, et al. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001;413:420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- Kim DC, Kim SH, Choi BH, Baek NI, Kim D, Kim MJ, et al. Curcuma longa extract protects against gastric ulcers by blocking H2 histamine receptors. Biol Pharm Bull. 2005;28:2220–2224. doi: 10.1248/bpb.28.2220. [DOI] [PubMed] [Google Scholar]
- Kim DH, Lee S, Chung YW, Kim BM, Kim H, Kim K, et al. Antiobesity and antidiabetes effects of a Cudrania tricuspidata hydrophilic extract presenting PTP1B inhibitory potential. BioMed Res Int. 2016;2016 doi: 10.1155/2016/8432759. 8432759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim OK, Nam DE, Jun W, Lee J. Cudrania tricuspidata water extract improved obesity-induced hepatic insulin resistance in db/db mice by suppressing ER stress and inflammation. Food Nutr Res. 2015;59:29165. doi: 10.3402/fnr.v59.29165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinker JF, Laugwitz KL, Hagelüken A, Seifert R. Activation of GTP formation and high-affinity GTP hydrolysis by mastoparan in various cell membranes. G-protein activation via nucleoside diphosphate kinase, a possible general mechanism of mastoparan action. Biochem Pharmacol. 1996a;51:217–223. doi: 10.1016/0006-2952(95)02119-1. [DOI] [PubMed] [Google Scholar]
- Klinker JF, Wenzel-Seifert K, Seifert R. G-protein-coupled receptors in HL-60 human leukemia cells. Gen Pharmacol. 1996b;27:33–54. doi: 10.1016/0306-3623(95)00107-7. [DOI] [PubMed] [Google Scholar]
- Konturek SJ, Obtułowicz W, Kwiecień N, Sito E, Oleksy J, Miszczuk-Jamska B. Effect of ranitidine, a new H2-antagonist, on gastric and pancreatic secretion in duodenal ulcer patients. Dig Dis Sci. 1980;25:737–743. doi: 10.1007/BF01345291. [DOI] [PubMed] [Google Scholar]
- Kowalsky SF, Hamilton RA, Figge HL. Drug usage evaluation: H2-receptor antagonist use in 30 hospitals. Hosp Formul. 1991;26:725–726. 732. 734–736 passim. [PubMed] [Google Scholar]
- Lee BW, Lee JH, Gal SW, Moon YH, Park KH. Selective ABTS radical-scavenging activity of prenylated flavonoids from Cudrania tricuspidata. Biosci Biotechnol Biochem. 2006;70:427–432. doi: 10.1271/bbb.70.427. [DOI] [PubMed] [Google Scholar]
- Lee H, Ha H, Lee JK, Seo CS, Lee NH, Jung DY, et al. The fruits of Cudrania tricuspidata suppress development of atopic dermatitis in NC/Nga mice. Phytother Res. 2012;26:594–599. doi: 10.1002/ptr.3577. [DOI] [PubMed] [Google Scholar]
- Moore M, Afaneh C, Benhuri D, Antonacci C, Abelson J, Zarnegar R. Gastroesophageal reflux disease: a review of surgical decision making. World J Gastrointest Surg. 2016;8:77–83. doi: 10.4240/wjgs.v8.i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa T, Hirata W, Higichi S. Effects of three H2-receptor antagonists (cimetidine, famotidine, ranitidine) on serum gastrin level. Int J Clin Pharmacol Res. 2002;22:29–35. [PubMed] [Google Scholar]
- Onodera S, Shibata M, Tanaka M, Inaba N, Arai Y, Aoyama M, et al. Gastroprotective mechanism of lafutidine, a novel anti-ulcer drug with histamine H2-receptor antagonistic activity. Arzneimittelforschung. 1999;49:519–526. doi: 10.1055/s-0031-1300454. [DOI] [PubMed] [Google Scholar]
- Park KH, Park YD, Han JM, Im KR, Lee BW, Jeong IY, et al. Anti-atherosclerotic and anti-inflammatory activities of catecholic xanthones and flavonoids isolated from Cudrania tricuspidata. Bioorg Med Chem Lett. 2006;16:5580–5583. doi: 10.1016/j.bmcl.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Repka-Ramirez MS. New concepts of histamine receptors and actions. Curr Allergy Asthma Rep. 2003;3:227–231. doi: 10.1007/s11882-003-0044-3. [DOI] [PubMed] [Google Scholar]
- Rieder F, Biancani P, Harnett K, Yerian L, Falk GW. Inflammatory mediators in gastroesophageal reflux disease: impact on esophageal motility, fibrosis, and carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2010;298:G571–G581. doi: 10.1152/ajpgi.00454.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana S, Kumar PP, Visweswaram D. Antiulcer activity of agnitundirasa and its comparison with cimetidine in shay rat. Anc Sci Life. 1989;8:207–211. [PMC free article] [PubMed] [Google Scholar]
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. quiz 1943. [DOI] [PubMed] [Google Scholar]
- Weiser HF, Gubernatis G, Siewert JR. Effect of oxmetidine and ranitidine on reflux behavior in patients with esophagitis. Z Gastroenterol. 1983;21:580–584. [PubMed] [Google Scholar]
- Xin LT, Yue SJ, Fan YC, Wu JS, Yan D, Guan HS, et al. Cudrania tricuspidata: an updated review on ethnomedicine, phytochemistry and pharmacology. RSC Advances. 2017;7:31807–31832. doi: 10.1039/C7RA04322H. [DOI] [Google Scholar]
- You Y, Min S, Lee YH, Hwang K, Jun W. Hepatoprotective effect of 10% ethanolic extract from Curdrania tricuspidata leaves against ethanol-induced oxidative stress through suppression of CYP2E1. Food Chem Toxicol. 2017;108:298–304. doi: 10.1016/j.fct.2017.08.007. [DOI] [PubMed] [Google Scholar]