Table 1.
Chemical Test of Leaving Group Activation for N-Ribosyltransferases*
| Enzyme | [kcat p-NO2-φ-riboside]/[kcat substrate] | |
|---|---|---|
| CfNH (IU-nucleoside hydrolase)a | 8.5 | 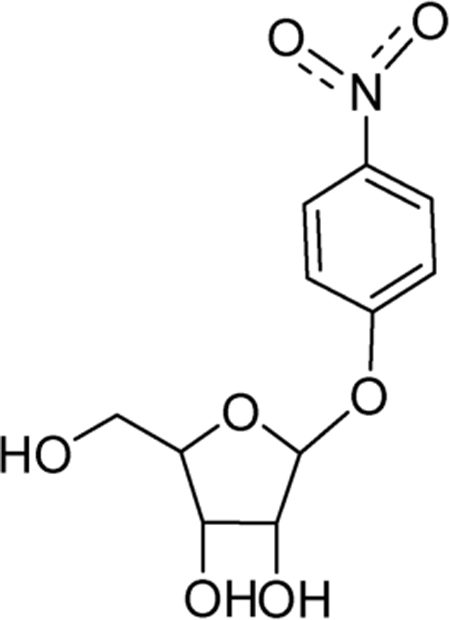 |
| LmNH (IU-nucleoside hydrolase)b | 1.8 | |
| IAG-nucleoside hydrolasec | 0.024 | |
| GI-nucleoside hydrolased | 0.0003 | |
| Purine nucleoside phosphorylasee | 0.000017 | |
| AMP nucleosidasef | 0.000015 | |
| Reactions compared p-nitrophenyl β-D-riboside as substrate to a,b,cinosine, dguanosine, and einosine. fp-Nitrophenyl β-D-riboside 5′-phosphate and AMP were compared as substrates for AMP nucleosidase. bLmNH is the enzyme from Leishmania major. Results from reference 89, 90. | ||
