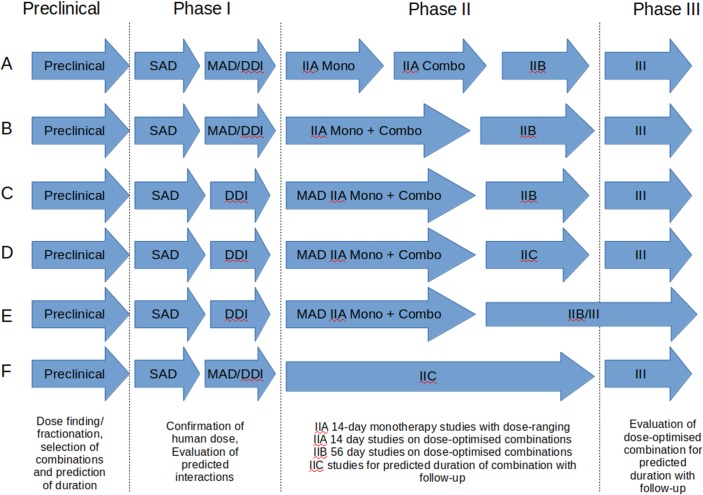
Fig 1.
Elements of critical pathways for clinical development of TB drugs illustrating selected alternatives: (A) Current standard approach, (B) 14+14 IIA design, (C) Dose-ranging 14+14 IIA design incorporating MAD PK in patients, (D) Phase IIC design, (E) Seamless IIB/III design, and (F) IIC design with no monotherapy (‘Mono’) stage. Combo, combination therapy; DDI, drug–drug interaction study; MAD, multiple ascending dose; PK, pharmacokinetic; SAD, single ascending dose; TB, tuberculosis.