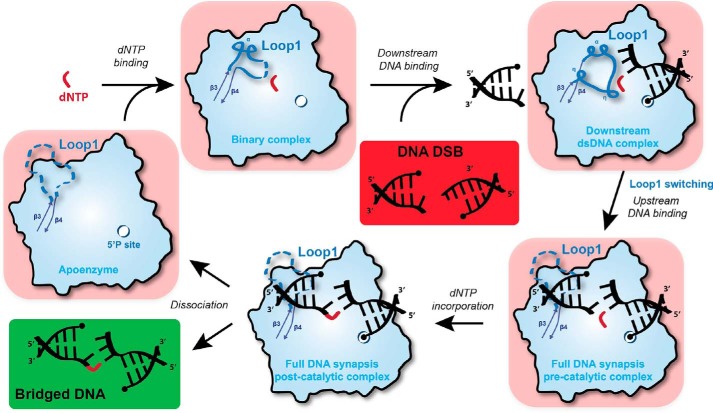
Figure 9.
Sequential model of Pol μ activity in the presence of a DSB DNA with 3′ protruding ends. Initially, a complex composed of free Pol μ (apoenzyme) and dNTP is formed. The binding of downstream dsDNA, strengthened by the 5′ phosphate-binding pocket, modifies Loop1 conformation to favor Watson–Crick interactions in the nascent bp but also prevents the binding of upstream dsDNA, including the primer. Subsequently, Loop1 is moved away, and the upstream dsDNA is recruited (DSB full synaptic complex), allowing nucleotide incorporation on the upstream primer (DSB post-catalytic complex). Finally, the enzyme and the bridged DNA dissociate to allow for the action of ligase IV. If the incoming dNTP does not form a Watson–Crick bp with the downstream template DNA end, then it is possible that the ternary complex (downstream duplex + dNTP-Pol μ) will disassemble. If the complex does not fall apart and the mismatched nucleotide is incorporated, then this would account for the low level of template-independent addition that is seen in Fig. 2C (bottom two boxes).