Abstract
Objective
The aim of this systematic review was to assess the efficacy and safety of pharmacological agents in the management of agitated behaviours following traumatic brain injury (TBI).
Methods
We performed a search strategy in PubMed, OvidMEDLINE, Embase, CINAHL, PsycINFO, Cochrane Library, Google Scholar, Directory of Open Access Journals, LILACS, Web of Science and Prospero (up to 10 December 2018) for published and unpublished evidence on the risks and benefits of 9 prespecified medications classes used to control agitated behaviours following TBI. We included all randomised controlled trials, quasi-experimental and observational studies examining the effects of medications administered to control agitated behaviours in TBI patients. Included studies were classified into three mutually exclusive categories: (1) agitated behaviour was the presenting symptom; (2) agitated behaviour was not the presenting symptom, but was measured as an outcome variable; and (3) safety of pharmacological interventions administered to control agitated behaviours was measured.
Results
Among the 181 articles assessed for eligibility, 21 studies were included. Of the studies suggesting possible benefits, propranolol reduced maximum intensities of agitation per week and physical restraint use, methylphenidate improved anger measures following 6 weeks of treatment, valproic acid reduced weekly agitated behaviour scale ratings and olanzapine reduced irritability, aggressiveness and insomnia between weeks 1 and 3 of treatment. Amantadine showed variable effects and may increase the risk of agitation in the critically ill. In three studies evaluating safety outcomes, antipsychotics were associated with an increased duration of post-traumatic amnesia (PTA) in unadjusted analyses. Small sample sizes, heterogeneity and an unclear risk of bias were limits.
Conclusions
Propranolol, methylphenidate, valproic acid and olanzapine may offer some benefit; however, they need to be further studied. Antipsychotics may increase the length of PTA. More studies on tailored interventions and continuous evaluation of safety and efficacy throughout acute, rehabilitation and outpatient settings are needed.
PROSPERO registration number
CRD42016033140
Keywords: traumatic brain injury, agitation, pharmacological intervention
Strengths and limitations of this study.
This systematic review assessed the efficacy and safety of pharmacological agents in the management of agitated behaviours following traumatic brain injury (TBI).
Randomised controlled trials, quasi-experimental and observational studies were reviewed.
The included studies were limited by small sample sizes, variations in the different agitated behaviours and populations studied.
The review found insufficient data to recommend the use of any agent for the management of agitated behaviours following TBI.
Introduction
Traumatic brain injury (TBI) occurs when an external force is applied to the head leading to alterations in brain function including decreased level of consciousness, post-traumatic amnesia (PTA) and changes in behaviour and cognition that can persist in the long term. In the USA alone, ~50 000 people die each year from TBI and >5 million live with TBI-related disabilities.1 2 While TBI has a substantial impact on direct healthcare costs, indirect costs from lost productivity also represent a significant economic burden.3 4 Agitated behaviours are a frequent behavioural problem following TBI.5 6 They have been broadly defined as a state of confusion that follows the initial injury and is characterised by disruptive behaviours. A constellation of behaviours has been associated with the term ‘agitation’ in TBI patients, including restlessness, confusion, physical and verbal aggression, impulsivity, perceptual disturbances and inattention creating a very heterogeneous group of patients to study.7 Agitation has been reported in 20%–41% of patients during the early stage of recovery in acute care units and up to 70% of patients in rehabilitation units.6 8–13 It can result in harm to patients and caregivers, interfere with treatments, lead to the use of physical and pharmacological restraints, increase hospital length of stay, delay rehabilitation and impede functional independence.10–12 14–16 In TBI outpatients, neurobehavioral symptoms may be different in nature. Aggressive behaviour and irritability, more than physical agitation are generally reported. A variety of agents such as antidepressants, anticonvulsants, stimulants and antipsychotics have been used for the management of neurobehavioral complications of TBI.17 18 However, preclinical studies have suggested that repeated use of certain agents such as haloperidol, risperidone and diazepam may reduce cognitive and functional recovery.19–22 Thus, it remains unclear which pharmacological agents are the most effective and safest for the management of agitated behaviours in TBI patients. A Cochrane Systematic Review published in 2006 showed a lack of evidence to support any agent.23 Since then, two additional systematic reviews concluded that the evidence was insufficient and too weak to recommend any specific agent; however, they included only French and English studies published before January 2016, had incomplete search strategies and did not include the grey literature.24 25 To advance this field, we updated and broadened the literature search, included all languages and included studies in which an agitated behaviour was not an eligibility criterion, but was measured as an outcome variable. The aim of this systematic review was to assess the efficacy and safety of pharmacological agents in the management of agitated behaviours following TBI compared with placebo or other treatments.
Methods
The review protocol has been registered in PROSPERO International Prospective Register of Systematic Reviews, conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines and published in a peer-reviewed journal.26 27 We included all randomised controlled, quasi-experimental and observational studies with control groups that had a majority (>50%) of patients with TBI. We excluded case reports, case series and observational studies without control groups. We included studies of all type of patients who suffered a TBI, including children and adults, in both the early stages of recovery and in rehabilitation. We included three mutually exclusive types of studies: (1) those evaluating the use of pharmacological interventions in which an agitated behaviour, not further defined, was one of the eligibility criteria for the study; (2) those in which an agitated behaviour was not an eligibility criterion, but was measured as an outcome variable; and (3) those specifically assessing the safety of pharmacological agents used to treat agitation in TBI patients. In this systematic review, we considered agitation, aggressiveness, assaultive behaviour, irritability and confusion as part of agitated behaviours. All medications considered in this review were prespecified and consisted in the following: beta-adrenergic blockers, typical and atypical antipsychotics, anticonvulsants, dopamine agonists, psychostimulants, antidepressants, alpha-2-adrenergic agonists, hypnotics and anxiolytics. Studies were included whether the investigators compared a medication to placebo, a medication to another medication or various combinations of different medications.
The primary outcome was a reduction in severity of the agitated behaviour as measured in each study. If feasible, we reported resolution of agitated behaviours as well as changes in duration and type of symptoms (confusion, aggressiveness, inattention, hallucinations, disorientation and inappropriate mood or speech). Secondary outcomes include lengths of stay (intensive care unit (ICU) length of stay, hospital LOS for the early rehabilitation phase), adverse events (extrapyramidal effects, QTc prolongation, cardiac arrhythmias, hypotension, seizures, behavioural effects), use of physical restraints in ICU, cognitive and functional outcomes at hospital discharge and at 1 year post-TBI.
Patient and public involvement
This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Search strategy
A search strategy was devised with the help of Health Sciences librarian (online supplementary file 1) and using the Peer Review for Electronic Search Strategies checklist was conducted in the following databases: PubMed, OvidMEDLINE, OvidMEDLINEIn-Process & Other Non-Indexed Citations, Embase, CINAHL, PsycINFO, Cochrane Library, Google Scholar, Directory of Open Access Journals, LILACS, Web of Science and Prospero (http://www.crd.york.ac.uk/PROSPERO/) up to 10 December 2018.28 A grey literature search was also performed using the resources suggested in CADTH’s Grey Matters (http://www.cadth.ca/en/resources/finding-evidence-is/grey-matters). As described in our published protocol, we searched abstracts from annual scientific meetings from relevant groups in the last 5 years.26 Finally, references of identified studies as well as other types of articles (reviews, book chapters) were screened.
bmjopen-2019-029604supp001.pdf (53.9KB, pdf)
Data collection and analysis
Two reviewers (DW, A-JF) independently screened titles and abstracts for eligible publications. The same reviewers then assessed the complete report of each retained citations for eligibility. Disagreements were resolved by consensus and discussion with a third reviewer was not required.
Data extraction and management
Data from all included studies were extracted by two independent reviewers (A-JF and DW) and in duplicate using a pretested data extraction form. The following variables were recorded for each study: study title, name of the first author, year of publication, country of origin, language of publication, publication type (journal article, conference proceeding, abstract, thesis), clinical setting (ICU), hospital ward, rehabilitation unit, outpatient), study design (randomised controlled, blinded or open, non-randomised controlled, prospective or retrospective, crossover), population (paediatric, adult), patient characteristics (age, gender, isolated TBI or multiple trauma including TBI, severity of TBI according to Glasgow Coma Scale, days from TBI at inclusion, inclusion and exclusion criteria), characteristics of the intervention and control treatment (type of pharmacological agent, dose, frequency and duration of the therapy), agitation measurement tool, description of the specific agitated behaviours (definition, frequency, duration) and clinical outcomes (length of stay), adverse events, use of physical restraints during ICU stay, duration of PTA, cognitive function at ICU discharge and at 1 year, and functional outcome at ICU discharge and at 1 year. We contacted the corresponding author for clarifications when necessary. In the case of an abstract not available in English, the research team included authors fluent in French, Spanish, German and Italian, who were able to read the abstract. Among selected articles, only one article in Spanish was included. The article was reviewed by authors fluent in Spanish.
Assessment of risk of bias
Two reviewers (DW, A-JF) independently evaluated each included study with the Cochrane Collaboration tool for randomised controlled trials and the Ottawa-Newcastle tool for observational studies, respectively.29 30 In case of disagreement concerning the risk of bias, a third reviewer (FB) was consulted to resolve the issue.
Results
Study selection
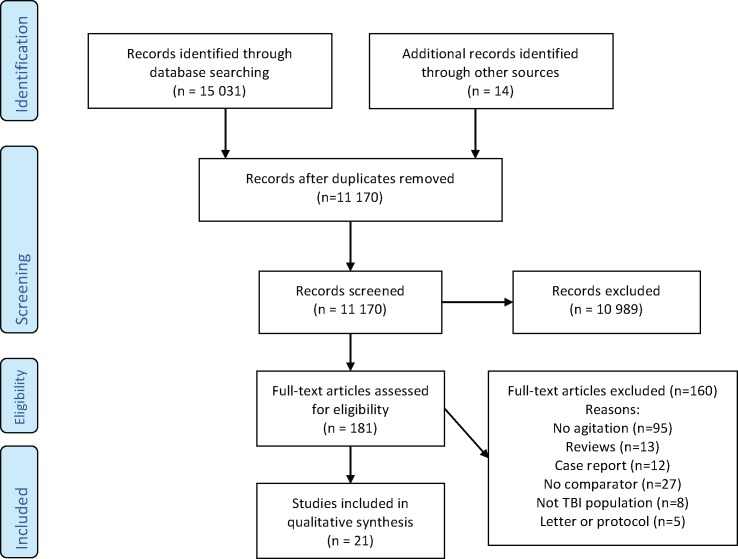
The database search (up to 10 December 2018) retrieved 11 170 unique citations of which 10 989 were excluded based on title and abstracts (figure 1). We assessed 181 full-text articles for eligibility and 21 studies were included. A total of eight studies evaluated the use of pharmacological interventions in which an agitated behaviour was the presenting symptom or one of the presenting symptoms.31–38 In nine other studies, agitated behaviour was not the presenting symptom, but was measured as an outcome variable.39–47 Finally, four studies specifically assessed the safety of pharmacological agents used for agitated behaviours in TBI.48–51
Figure 1.
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis; TBI, traumatic brain injury.
Agitated behaviours as the presenting symptom
The eight included studies evaluated various aspects ranging from aggressiveness to irritability and confusion (table 1).31–38 The behaviours were evaluated using the following tools (table 2): Agitated Behaviour Scale (ABS), confusion assessment protocol, State-Trait Anger Scale, the overt aggression scale, Richmond Agitation Sedation Scale and neuropsychiatric inventory irritability (NPI-I) and neuropsychiatric inventory aggression domains.52
Table 1.
Study characteristics
| Study/year (N) |
Publication/country | Study design | Study focus/population | Interventional arm/population | Comparative arm/population | Location at randomisation | Timing from TBI at randomisation | TBI description |
| 1. Agitated behaviour as the presenting symptom | ||||||||
| Brooke, et al
31/1992 (n=21) |
Published USA |
RCT parallel | Agitation Mean age: 31 87 men and 13 women |
Propranolol 60–420 mg/day | Placebo | Level 1 trauma and rehabilitation centre | N/A | Severe blunt TBI |
| Mooney, Hass32/1993 (n=38) |
Published USA |
Randomised Pre–post |
Anger Mean age: 29±10 Male gender: 100% |
Methylphenidate 30 mg/day | Placebo | Outpatient | 6 months or more (mean 27±21 months) | Severe blunt TBI |
| Yablon, et al
33/2010 (n=79) |
Abstract USA |
RCT parallel | Confusion Age and gender not reported |
Amantadine 100 mg twice daily × 14 days |
Placebo | Inpatient brain injury unit of a rehabilitation hospital | ≤6 months | TBI not further defined |
| Hammond, et al
34/2014 (n=76) |
Published USA |
RCT parallel | Irritability and aggression | Amantadine 100 mg twice daily Mean age: 40±13 Male gender: 74.4% |
Placebo 38±12 Male gender: 80.5% |
Outpatient | ≥6 months following a TBI | Blunt TBI |
| Beresford, et al
37/2015 (n=50) |
Abstract USA |
RCT parallel | Agitation Mean age: 47±14 46 men and 4 women |
Valproic acid for level 50–100 μg/mL | Placebo | Outpatient | >1 year following TBI | Mild and moderate TBI |
| Hammond, et al
35/2015 (n=168) |
Published USA |
RCT parallel | Irritability and aggression | Amantadine 100 mg twice daily Mean age: 40±13 Male gender: 80.5% |
Placebo Mean age: 38±12 Male gender: 74.4% |
Outpatient | ≥6 months following a TBI | Blunt TBI |
| Maturana Waidele, Maturana Rodillo38/2009 (n=31) |
Published Chile |
Prospective double-blind | Restlessness, irritability, aggression, insomnia Age and gender not reported |
Olanzapine (dose not specified) | Placebo | Outpatient | N/A | TBI not further defined |
| Gramish, et al
36/2017 (n=139) |
Published USA |
Retrospective observational | Agitation | Amantadine 100 mg twice daily Mean age: 42±17 Male gender: 81.4% |
No amantadine Mean age: 48±21 Male gender: 76.8% |
Adult trauma ICU | Acute TBI | TBI not further defined |
| 2. Agitated behaviour is not the presenting symptom | ||||||||
| Study/year (N) |
Publication/country | Study design | Study focus | Interventional arm | Comparative arm | Location at randomisation | Timing from TBI at randomisation | TBI description |
| Schneider42/1999 (n=10) |
Published USA |
RCT parallel | Cognitive function and behaviour Mean age: 31 7 men and 3 women |
Amantadine 50 mg twice daily increased to 150 mg twice daily | Placebo | Outpatient | N/A | Moderate and severe TBI |
| Meythaler, et al
41/2001 (n=9) |
Published USA |
RCT crossover | Recovery and arousal Age and gender not reported |
Sertraline | Placebo | Inpatient rehabilitation | <2 weeks of TBI | Severe TBI |
| Meythaler, et al
43/2002 (n=35) |
Published USA |
RCT crossover | Neurological recovery Mean age: 31 26 men and 9 women |
Amantadine | Placebo | Emergency department | Between 4 days and 6 weeks following TBI | Severe blunt TBI |
| Banos, et al
39/2010 (n=99) |
Published USA |
RCT parallel | Cognitive function and behaviour | Sertraline Mean age: 35±17 Male gender: 79% |
Placebo Mean age: 35±16 Male gender: 66% |
Level 1 trauma centre inpatients | <8 weeks of TBI | Moderate and severe TBI |
| Giacino, et al
40/2012 (n=184) |
Published USA, Denmark, Canada |
RCT parallel | Functional recovery | Amantadine Mean age: 35±15 Male gender: 74% |
Placebo Mean age: 37±15 Male gender: 71% |
Inpatients | 4–16 weeks following TBI | Vegetative or minimally conscious TBI |
| Tramontana, et al
44/2014 (n=22 but 13 completed the study) |
Published USA |
RCT crossover | Attention Mean age: 29±9 Male gender: 69% |
Lisdexamfetamine | Placebo | Outpatient | 6–34 months (mean 15.6+/-10 months) since TBI | Moderate and severe TBI |
| Johansson, et al
46 2014 (n=24) |
Published Sweden |
RCT Crossover | Mental fatigue and cognition Mean age 39±11 Male gender: 50% |
Methylphenidate 5 mg and 20 mg tid |
Placebo | Outpatient | >12 months following TBI | Mild or moderate TBI |
| Fann, et al
45/2017 (n=62) |
Published USA |
RCT parallel | Major depression | Sertraline Mean age: 38±12 Male gender: 74% |
Placebo Mean age: 37±13 Male gender: 77% |
Level 1 trauma centre | <1 year of TBI | Moderate and severe TBI |
| Hart, et al
47/2017 (n=32) |
Published USA |
RCT parallel | Cognitive function | Dextroamphetamine Mean age: 39±16 Male gender: 65% |
Placebo Mean age: 39±18 Male gender: 100% |
TBI rehabilitation unit | <6 months of TBI | Moderate and severe TBI |
| 3. Studies assessing the safety of pharmacological agents used for agitated behaviours in TBI | ||||||||
| Rao, et al
50/1985 (n=26) |
Published USA |
Retrospective observational | Rehabilitation outcomes | Haloperidol Median age: 34 Gender not reported |
No haloperidol Median age: 22 Gender not reported |
Trauma and rehabilitation centre | From admission | Severe closed head injury |
| Mysiw, et al
49/2006 (n=182) |
Published USA |
Retrospective cohort | Cognitive and motor recovery Mean age: 36 Male gender: 74% |
Narcotics, benzodiazepines and neuroleptics | No CNS active medications | Level one trauma centre and rehabilitation centre | From admission | TBI |
| Kooda, et al
51/2015 (n=195) |
Abstract USA |
Retrospective observational | Duration of post-traumatic amnesia Age and gender not reported |
Antipsychotics | No antipsychotic | Level one trauma centre and rehabilitation centre | From admission | TBI |
| Anderson, et al
48/2016 (n=101) |
Published USA |
Retrospective cohort | Seizures, neuroleptic malignant syndrome, QTc prolongation, extrapyramidal symptoms, haematological disturbances | Haloperidol Median age: 32 Male gender: 87% |
No haloperidol Median age: 47 Male gender: 61% |
Inpatients | From admission | Moderate and severe TBI |
CNS, Central Nervous System; RCT, Randomized controlled trial; TBI, traumatic brain injury.
Table 2.
Tools used to measure agitated behaviours
| Tools | Description |
| Agitated Behaviour Scale74 | Scale of 14 items with 4 levels of scoring to assess the nature and extent of agitation during the acute recovery of traumatic brain. Total scores >21 are considered as agitation. |
| Brief Anger and Aggression Scale75 | A six-item measure developed for the rapid screening and identification of anger and aggression levels. |
| Confusion assessment protocol76 | Combination of orientation, cognition and other clinical measures of early confusion following traumatic brain injury. |
| Functional independence measure77 | Functional assessment measure with a 18-item ordinal scale used in the rehabilitation population. It offers a useful assessment of patient progress during inpatient rehabilitation. |
| Global improvement subscale of the Clinical Global Impressions (CGI)78 | The CGI is a 3-item observer-rated scale that measures illness severity (CGIS), global improvement or change (CGIC) and therapeutic response. |
| Belligerence cluster score for the Katz adjustment scale (KAS)79 | The KAS is an observer rating scale used to assess the social adjustment of people with traumatic brain injury. |
| Neuropsychiatric inventory irritability (NPI-I) and aggression domains (NPI-A)52 | The NPI is a 40-item scale evaluating 12 behavioural domains including irritability and aggression. The NPI-I items include bad temper, rapid mood changes, sudden anger, impatience, crankiness and argumentative. Raters evaluate frequency and severity of behaviours in the last month. The NPI aggression domain assesses the tendency to get upset, resistance to activities, stubbornness, uncooperativeness, shouting, cursing and physical behaviours indicative of aggression. The NPI score is the product of frequency and severity. The worst item score provided by the scorer is NPI-I or NPI-A most aberrant. |
| Neurobehavioral Function Inventory (NFI)80 | The NFI provides information on the frequency of behaviours and symptoms commonly associated with brain injury. Two versions of the NFI are available, one for completion by family members, another for completion by the person with the injury. |
| Neurobehavioral rating scale (NRS)81 | The NRS is a 28-item observer-rated instrument that measures a broad range of cognitive and noncognitive symptoms. It measures symptoms associated with psychiatric disorders as well as cognitive impairment and behavioural disturbances. |
| Overt aggression scale (OAS)82 | Scale for the objective rating of verbal and physical aggression. The OAS measures aggressive behaviours divided into 4 categories: verbal aggression, physical aggression against objects, physical aggression against self and physical aggression against others. |
| Anger-Hostility factor score of the Profile of Mood States (POMS)32 | The POMS consists of 65 adjectives that describe moods or feelings, to which the patient responds on a 5-point scale that ranges from ‘Not at all’ to ‘Extremely’. The POMS measures six identifiable mood/affective states: tension-anxiety, depression-dejection, anger-hostility, vigor-activity (V); fatigue-inertia (F) and confusion-bewilderment (C). |
| State-Trait Anger Scale (STAS)32 | The STAS is a 20-item self-report scale assessing two types of anger (State and Strait). State anger is comprised of tension, annoyance, irritability or rage. Whereas trait anger is the frequency with which a person feels state anger over time. |
Of the identified studies, two were conference abstracts that remained unpublished.33 37 The studies evaluated propranolol,31 amantadine,33–35 methylphenidate,32 valproic acid37 and olanzapine38 in comparison to placebo. Five used a randomised controlled parallel design,31 33–35 37 one used a randomised pretest posttest control group design,32 one was a prospective double blind observational study38 and, one was a retrospective observational study.36 All the studies exclusively enrolled adult (16 years or older) TBI patients and three studies excluded older patients (>65 or 75 years).34 35 37 The studies mostly included patients in rehabilitation (n=2)31 33 and outpatient (n=5) settings.32 34 35 37 38 Only one study evaluated patients in an ICU setting.36 All the studies exclusively studied TBI patients.31–38 Three studies identified in an earlier systematic review were excluded (figure 1) because TBI patients represented <50% of the sample.23 53–55
In the eight studies, one randomised trial evaluated the use of propranolol for the treatment of agitation in severe blunt TBI patients (table 3).31 It reported a reduction in the intensity of agitation episodes and in the use of physical restraints but failed to show a reduction in the frequency of agitation episodes.31 Amantadine was evaluated for the management of confusion in a randomised trial, irritability in two randomised trials and agitation in a retrospective observational study.33–36 The studies reported inconsistent results (table 3). In one unpublished study in the setting of rehabilitation within 90 days of TBI (n=79), amantadine had no effect on confusion.33 In a pilot study of outpatients who suffered a TBI >6 months ago, amantadine showed significant reductions in irritability and aggression using the Neuropsychiatric Inventory scale (NPI).35 In a follow-up study of 168 outpatients who had suffered a TBI >6 months ago, no difference in the incidence of irritability at 28 and 60 days using the NPI-I from observers (family member, close friend or employer) was reported.34 Participants self-rating at day 60 indicated improvement in irritability (p<0.04) but the difference became non-significant when adjusted for multiple comparisons. The Global improvement subscale of the Clinical Global Impressions (CGI), which evaluates general emotional and behavioural function, improved more in the amantadine group than in the placebo group at day 60 (p=0.0354). A subanalysis of patients with anger and aggression (118 of the 168 patients) in the same study was also carried out and reported a statistically significant reduction in participant’s self-rated aggression at 60 days.56 Finally, in a retrospective observational study (n=139), patients exposed to amantadine in the ICU reported more agitation episodes defined as a Richmond Agitation Sedation Score of +2 or higher (38% vs 14%) in an unadjusted analysis.36 The use of amantadine was also associated with an increased median ICU length of stay (4.5 vs 3 days; p=0.01) when compared with non-exposed patients.
Table 3.
Efficacy and safety outcomes
| Study /year/n |
Intervention | Agitated behaviour measures | Efficacy outcomes | Safety outcomes |
| 1. Agitated behaviour as the presenting symptom | ||||
| Randomised controlled studies | ||||
| Brooke, et al
31
/1992/n=21 |
Propranolol | Overt aggression scale | Significant reduction in maximum intensities of agitation per week (p<0.05). No significant difference in average number of agitation episodes per week. Significant reduction in physical restraint use during the study (p<0.05). | No safety outcomes reported |
| Mooney, Haas32
/1993/n=38 |
Methylphenidate | State-Trait Anger Scale, Belligerence cluster score for the Katz adjustment scale and the Anger-Hostility factor score, Organic Signs and Symptoms Inventory | Significant difference in the comparison of methylphenidate and placebo group on all the anger measures before and after 6 weeks in a multivariate analysis (p=0.02). | No significant effect on side effects |
| Yablon, et al
33
/2010/n=79 |
Amantadine | Confusion assessment protocol (CAP) | No significant differences in the number of symptoms of post-traumatic confusional state as measured by the CAP at 14 days (amantadine 2.56 vs placebo 2.7; p=0.57). Mean difference in time to first ‘non-confused’ CAP score between groups approached significance (amantadine 7.7 days and placebo 9.3 days; p=0.053). | No patients withdrawn because of safety criteria |
| Hammond, et al
35
/2014/n=76 |
Amantadine | NPI-I most aberrant and most problematic Irritability (NPI-I) and aggressiveness (NPI-A) |
Significant reduction in irritability (80.56% improved at least three points on the NPI-I, compared with 44.44% in the placebo group; p=0.0016). Mean change in NPI-I was −4.3 in the amantadine group and −2.6 in the placebo group (p=0.0085). When excluding individuals with minimal to no baseline aggression, mean change in NPI-A was −4.56 in the amantadine group and −2.46 in the placebo group (p=0.046). | No difference in adverse events (tremors, appetite, gastrointestinal, aches and pain, sexual problems, disorientation, seizures) |
| Beresford, et al
37
/2015/n=50 |
Valproic acid | Agitated Behaviour Scale by spouse or significant other | Significant others' weekly Agitated Behaviour Scale ratings were statistically lower, indicating less agitation in the valproic acid group, 12.9±4.9, than in the placebo group, 15.5±6.6, with significance at p=0.0367. | No safety outcomes reported |
| Hammond, et al
34
/2015/n=168 |
Amantadine | NPI-I most problematic by observer and by patient. Global improvement subscale of the Clinical Global Impressions (CGI) by physicians | Observer ratings were not different at day 28 or 60. Participants rating at day 60 showed improvement in NPI-I most problematic (p<0.04; but NS for when adjusted for multiple comparisons). Physician’s assessment of global improvement improved more in the amantadine group than the placebo group at 60 days (p=0.0354). | Well tolerated with no significant differences in adverse events between groups |
| Observational studies | ||||
| Maturana Waidele, Maturana Rodillo38
/2009/n=31 |
Olanzapine | Restlessness, irritability, aggressiveness and insomnia. No tool mentioned | Reduction in irritability (p<0.001), aggressiveness (p=0.008) and insomnia (p=0.011) between weeks 1 and 3 in the patients treated with olanzapine. | No safety outcomes reported |
| Gramish, et al
36
/2017/n=139 |
Amantadine | RASS score of +2 or higher | Increase in agitation in patients exposed to amantadine (38%) compared with non-exposed (14%); p=0.018. Increase in median ICU length of stay (4.5 vs 3 days; p=0.01). Median hospital length of stay was non-significantly increased (14 days vs 10 days; p=0.051). | No safety outcomes reported |
| 2. Agitated behaviour is not the presenting symptom | ||||
| Randomised controlled studies | ||||
| Schneider, et al
42
/1994/n=10 |
Amantadine | Neurobehavioral rating scale | No significant difference in behaviour scores between amantadine and placebo groups. | No safety outcomes reported |
| Meythaler, et al
41
/2001/n=9 |
Sertraline | Agitated Behaviour Scale | No difference in decline of ABS over treatment period | No safety outcomes reported |
| Meythaler, et al
43
/2002/n=35 |
Amantadine | Agitated Behaviour Scale | There were no statistically significant changes or trends in the ABS during the first 6 weeks or the second 6 weeks of the study (p>0.05, Mann-Whitney U test). | No detrimental effects in haematology or biochemistry laboratories and no seizures |
| Baños, et al
39
/2010/n=99 |
Sertraline | Aggression self-report and family report according to the Neurobehavioral Function Inventory | No significant differences between sertraline and placebo in patient self-report and family report. | No safety outcomes reported |
| Giacino, et al
40
/2012/n=184 |
Amantadine | Agitation and restlessness not further defined | A total of 12/87 (14%) patients and 11/97 (11%) patients exposed to amantadine and placebo developed agitation (p=NS) over the 4-week period. Restlessness was reported in 8% and 9% of patients exposed to amantadine and placebo, respectively. | No differences in adverse events (seizure, nausea, vomiting, constipation, diarrhoea, elevated liver function tests, insomnia, rash, congestive heart failure, involuntary muscle contractions) |
| Tramontana44
/2014/n=22 but 13 patients completed the study |
Lisdexamfetamine | Agitation and restlessness not further defined | No difference in agitation (no cases in each group) or irritability (1/13 case) during placebo) between the lisdexamfetamine and placebo groups. | Reduced appetite and weight loss of >5 lbs more frequent with lisdexamfetamine (7 vs 1 case) p=NS |
| Johansson46
/2014/n=48 |
Methylphenidate | Aggression, restlessness and irritability not further defined | No difference in aggression, restlessness and irritability in patients treated with methylphenidate. | A significant increase in heart rate was found. No significant changes were found in blood pressure or QT intervals |
| Fann45
/2017/n=62 |
Sertraline | Brief Anger and Aggression Scale and agitation/restlessness not further defined | No difference in the Anger and Aggression Scale. More patients developed agitation/restlessness in the sertraline group (17%) versus the placebo group (7%) p=0.42. | No significant difference in safety outcomes. More patients in the sertraline group (17%) developed gas/flatulence versus the placebo group (0%) p=0.052 |
| Hart47
/2017/n=32 |
Dextroamphetamine | Agitated Behaviour Scale | Increase in agitation with dextroamphetamine over time compared with placebo (p<0.05). | No significant difference in heart rate or blood pressure |
RASS, Richmond Agitation Sedation Scale.
The efficacy of olanzapine in the management of restlessness, irritability, aggression and insomnia in outpatients with a history of TBI was evaluated in a prospective double blind study.38 While no reduction in restlessness was reported, the authors did report a significant reduction in irritability and insomnia between weeks 1 and 3 in olanzapine-treated patients. Unfortunately, no statistical comparison with the placebo group was provided. The efficacy of valproic acid in reducing agitated behaviours among mild and moderate TBI outpatients was evaluated in an unpublished randomised controlled study (n=50).37 Patients were included >1 year following brain injury and suffered from both affective lability and alcohol dependence. A significant reduction in the ABS evaluated by family members at eight weeks (12.9 vs 15.5 281 points; p=0.03) was observed. Finally, a crossover study assessed methylphenidate for 282 anger (n=38) in TBI rehabilitation centre outpatients (6 months or more after TBI). After 283 6 weeks, methylphenidate significantly reduced the anger score using the State Trait 284 Anger Scale (STAS).32 Of the eight studies, safety outcomes were reported in four studies.32–35 When reported, the agents studied were well tolerated with no significant differences observed. Functional and cognitive outcomes were not reported in any of these studies.
Agitated behaviour as a secondary measure
We identified nine studies evaluating agitated behaviours as a secondary measure, which were focused on cognitive function and neurological recovery (table 1).39–47 In these studies, sertraline,39 41 45 amantadine,40 42 43 amphetamines44 47 and methylphenidate46 were evaluated versus placebo and reported agitated behaviours as an outcome. Of these studies, six used a randomised crossover design and three used a randomised controlled parallel design.
Sertraline was evaluated in three studies to enhance recovery and increase arousal, ameliorate cognitive and neurobehavioral functioning and to treat major depression (table 3).39 41 45 In all these three studies, sertraline had no effect on the incidence of agitation, anger or aggression. In one study, more patients developed agitation/restlessness in the sertraline group (17%) compared with the placebo group (7%) but this difference was not statistically significant (p=0.42).45 Amantadine was also evaluated in three studies for cognitive and functional recovery.40 42 43 All three studies found no differences in agitated behaviours compared with placebo. Methylphenidate was evaluated for secondary mental fatigue in mild TBI patients >6 months after injury.46 However, it had no effect on irritability and aggression. Lisdexamfetamine and dextroamphetamine were each evaluated for attention deficits in TBI patients and no effect on agitated behaviours was noted with lisdexamfetamine whereas dextroamphetamine increased agitation over time (p<0.05).44 47 Among these nine studies, those evaluating sertraline and amantadine reported no significant differences in adverse events.39–43 45
Studies evaluating safety outcomes
Finally, the safety of pharmacological agents used for agitated behaviours in TBI patients was evaluated in four retrospective observational studies (table 4).48–51 Two of these studies focused on the effect of haloperidol and antipsychotic use on PTA duration, whereas a third evaluated the effects of antipsychotics, benzodiazepines and narcotics on PTA duration, and Functional independence measure (FIM) cognitive and motor scores.49–51 In these three studies, haloperidol and other antipsychotics were associated with an increase in PTA duration. Antipsychotics, benzodiazepines and narcotics had no effects on FIM scores.49 Finally, a fourth study focused on the general safety (seizures, neuroleptic malignant syndrome, QTc prolongation, extrapyramidal symptoms, haematological disturbances) of haloperidol in ICU TBI patients.48 Patients exposed to haloperidol (n=45) had no significant increase in adverse events compared with non-exposed patients (n=56). Of note, none of the studies adjusted for severity of TBI and other potential confounders.
Table 4.
Studies assessing the safety of pharmacological agents used for agitated behaviours in TBI
| Study/year/n | Drugs studied | Results |
| Rao50/1985/n=26 | Haloperidol | Twenty-five patients exhibited agitation and 11 patients required haloperidol. In an unadjusted analysis, the haloperidol patients have a significantly longer period (8 vs 4 weeks; p<0.03) of post-traumatic amnesia (PTA). |
| Mysiw49/2006/n=182 | Narcotics, benzodiazepines and neuroleptics | Narcotics, benzodiazepines and neuroleptics had no effect on the Function Independence Measures (FIM) motor and independence scores. In an unadjusted analysis, narcotics and neuroleptics increased duration of PTA by >7 days (p<0.01). |
| Kooda51
/2005/n=195 |
Antipsychotics | Fifty-two patients received antipsychotics (26.7%) within 7 days of TBI, mostly quetiapine. In an unadjusted analysis, duration of PTA was significantly longer (19.6 vs 12.3 days; p=0.013) in patients treated with antipsychotics. |
| Anderson48
/2016/n=101 |
Haloperidol | In an unadjusted analysis, there was no significant increase in adverse events (QT prolongation, seizures, neuroleptic malignant syndrome, extrapyramidal symptoms or haematological disturbances) associated with haloperidol use. Patients in the haloperidol group who developed complications received a higher mean daily dose (p=0.013). There was no difference in length of mechanical ventilation but the haloperidol group had a longer hospital length of stay (22 vs 11 days; p<0.001). |
TBI, traumatic brain injury.
Risk of bias assessment
Risk of bias scores are reported in table 5. The analysis of risk of bias of randomised controlled trials with the Cochrane Collaboration’s Tool revealed that many studies did not provide sufficient information on sequence, generation and allocation concealment. A majority of studies had other threats to validity including limited sample sizes, no description of patient demographics and loss to follow-up. For six studies evaluated with the Newcastle-Ottawa tool, the number of stars awarded ranged from 4 to 5. Most studies were awarded a score of 4 stars, indicating a high risk of bias. As none of the six studies were adjusted for potential confounding, all received 0 stars for comparability.
Table 5.
Risk of bias assessment
| 1. Randomised controlled trials | |||||||
| Cochrane Collaboration Tool Risk of bias items | |||||||
| Study (year) | Sequence generation | Allocation | Blinding of participants and personnel | Blinding of outcome assessment | Outcome data | Selective reporting | Other threats to validity |
| Brooke16 (1992) | U | U | L | L | L | L | H |
| Mooney32 (1993) | U | U | L | H | L | U | H |
| Schneider42 (1999) | U | U | U | U | H | L | H |
| Meythaler41 (2001) | U | U | L | L | U | U | H |
| Meythaler43 (2002) | U | U | U | U | L | H | H |
| Baños39 (2010) | U | U | L | L | L | L | H |
| Yablon33 (2010) | U | U | L | L | L | U | H |
| Giacino40 (2012) | U | L | L | L | L | L | L |
| Hammond35 (2014) | L | L | L | L | U | L | L |
| Tramontana44 (2014) | H | H | L | L | H | L | H |
| Johansson46 (2014) | U | H | H | H | H | L | H |
| Beresford37 (2015) | U | U | L | L | H | L | H |
| Hammond17 (2015) | L | L | L | L | U | L | L |
| Fann45 (2017) | L | L | L | L | L | L | H |
| Hart47 (2017) | U | U | L | L | L | L | L |
| 2. Observational studies | |||
| Study (year) | Newcastle-Ottawa Quality Assessment Scale No of stars awarded |
||
| Selection* | Comparability† | Outcome‡ | |
| Rao50 (1985) | ** | ** | |
| Maturana Waidele38 (2009) | ** | ** | |
| Mysiw49 (2006) | ** | *** | |
| Kooda51 (2015) | ** | ** | |
| Anderson48 (2016) | ** | ** | |
| Gramish36 (2017) | *** | * | |
For Cochrane Collaboration’s Tool: For Newcastle-Ottawa Quality Assessment Scale.
*Maximum four stars.
†Maximum two stars.
‡Maximum three stars.
H, high risk of bias; L, low risk of bias; U, unclear risk of bias.
Discussion
In this systematic review, we used an exhaustive search strategy and included studies directly or indirectly evaluating pharmacological agents for the management of TBI-associated agitated behaviours as well as studies assessing the safety of pharmacological agents used for these agitated behaviours. Despite the prevalence and importance of this problem, we found a limited number of studies evaluating pharmacological interventions for the management of agitated behaviours. Propranolol, methylphenidate, valproic acid and olanzapine were the only agents suggesting a potential benefit in reducing agitation, anger or irritablility.31 32 37 38 However, the studies evaluating these agents had limited sample sizes, heterogeneous patient populations and an unclear risk of bias. Amantadine showed mixed results whereas sertraline, lisdexamfetamine and dextroamphetamine showed no benefits. In comparison to the two most recent systematic reviews, we used a more rigorous and broader search strategy. As such, we restricted our search to randomised controlled, quasi-experimental and observational studies with control groups that had a majority (>50%) of patients with TBI, thus excluding case reports, case series and uncontrolled observational studies. Our updated and broadened literature search enabled the identification of two additional studies from the grey literature, three recently published studies and one non-English study.24 25 33 36 37 45 47 Our search strategy also included studies evaluating agitated behaviours as a secondary measure and identified nine more studies, thus adding to previous systematic reviews. Furthermore, we included studies where the safety of pharmacological agents for the management of agitated behaviours was assessed and identified four such studies.
The use of beta-blockers in patients with organic brain disease and assaultive behaviours or impulsivity has been previously studied in three crossover-randomised trials with some efficacy but TBI represented <50% of the total patient population.53–55 In the study presented in this review, propranolol reduced the intensity of agitation but not the frequency.31 One important finding was a reduction in the use of physical restraints. Unfortunately, safety measures such as hypotension and bradycardia were not reported.
The Canadian ABIKUS (Acquired Brain Injury Knowledge Uptake Strategy) guidelines have recommended beta-blockers for the treatment of aggression following TBI.57
Although numerous observational studies have reported a reduction in agitation with the use of antipsychotic agents, we found no controlled studies evaluating the efficacy of antipsychotics other than olanzapine.58–60 In a previous systematic review that included case reports and case series evaluating antipsychotics, Planthier et al identified seven articles that included a total of 52 patients.24 The lack of a control group excluded these studies from our review. The only study we included that used olanzapine did not report a reduction in restlessness but did suggest a reduction in irritability.38 Its interpretation is greatly limited given the poor description of methods and a lack of statistical comparison with the placebo group. The four studies assessing safety all evaluated antipsychotic agents and suggested a potential risk of prolonged PTA in unadjusted analyses.48–51 None of the studies controlled for potential confounders such as severity of TBI. Although preclinical studies have suggested a reduction in cognitive and motor recovery with repeated administration of haloperidol and risperidone, the one study evaluating cognitive and motor scores reported no significant association with antipsychotic use.19–21 49 61 In light of these results, both the International Cognitive, the Canadian ABIKUS guidelines and the French Society of Physical and Rehabilitation Medicine guidelines have advised against the use of antipsychotics in TBI patients with agitated behaviours.24 57 62 Paradoxically, observational studies have suggested antipsychotics are frequently used for the management of agitated behaviours.14 63–65
Anticonvulsants are clinically used as mood stabilisers in bipolar affective disorder and have also been used in TBI-associated agitation.66 67 Case series have reported a reduction in agitation and aggressive behaviours with the use of valproic acid and carbamazepine but were uncontrolled.68–72 We identified one unpublished study of TBI patients with affective lability and alcohol dependence where valproic acid showed effectiveness in reducing weekly ABS rated by spouse or significant other’s. Unfortunately, the abstract provided no information on the onset of effect or adverse events associated with its use.
Amantadine increases dopaminergic neurotransmission and has been shown to increase the rate of neurological recovery in severe TBI.40 In the four studies that evaluated amantadine for irritability, agitation or aggressiveness, results were variable.33–36 Although one study suggested a reduction in irritability in outpatients, a larger study by the same group failed to confirm these results.34 35 Interestingly, a recent observational study of patients exposed to amantadine in the ICU reported an increased risk of agitation.36 Although these effects were not observed in a multicentre trial that started amantadine at least 4 weeks after TBI, the early use of amantadine in the ICU may explain these findings.36 40 However, these results were uncontrolled and confounding may also explain these differences. In addition, the use of amantadine may have increased arousal and the agitation measured may be part of the natural recovery. In studies in which agitation was not the presenting symptom, no significant differences in behaviour scores between amantadine and control groups were reported.40 42 43
In this review, we found no comparative studies assessing the efficacy of tricyclic antidepressants, dexmedetomidine or benzodiazepines. We also found no studies in children. A search of TBI-associated agitation studies in clinical trial registries revealed ongoing studies with the combination of dextromethorphan and quinidine (ClinicalTrials.gov: NCT03095066) as well as propranolol and clonidine (ClinicalTrials.gov: NCT01322048).73 Finally, in a recent observational study on the predictors of agitation in TBI rehabilitation, sodium channel antagonist anticonvulsants, second-generation antipsychotics and gamma-aminobutyric acid anxiolytics were associated with more severe agitation.14 Although indication bias and residual confounding are probable, these results do suggest an association between suppression of cognition and more agitation.
Strengths of this study include an exhaustive search of the literature in the adult and paediatric populations, including grey literature and no language limitation. A risk of bias assessment was performed for each included study. Limits of this study include the presence of significant heterogeneity, variations in the different agitated behaviours (agitation, irritability and aggression) and populations (acute TBI, rehabilitation, outpatient) evaluated, preventing the authors from proceeding to a meta-analysis. In addition, very little studies reported length of stay and functional outcomes.
Conclusion
In conclusion, there are insufficient data to recommend the use of any medications for the management of agitation following TBI. Propranolol, methylphenidate, valproic acid and olanzapine may offer some benefit; however, they need to be further studied. The use of amantadine in the acutely ill may increase the risk of agitation whereas antipsychotics may prolong PTA. More studies on tailored interventions and continuous evaluation throughout the acute, rehabilitation and outpatient settings are needed to assess the efficacy and safety of pharmacological agents for the management of agitated behaviours in both the adult and paediatric TBI populations. In addition, there is a need to better define and standardise the assessment of agitated behaviours. Newer agents such as dexmedetomidine should also be evaluated.
Supplementary Material
Acknowledgments
We thank M Patrice Dupont, librarian at the Université de Montréal for his expertise and help with the literature search strategies.
Footnotes
Contributors: DW, A-JF, LDB, MP, EC, FL, M-JP, J-FG, SM and FB participated in the design, writing of the the review protocol and contributed to the final manuscript. DW wrote the search strategy and undertook the literature search. DW, A-JF and FB conducted the title and abstract screening and full article screening for final study inclusion. DW and A-JF conducted data collection and cleaning, LB, MP and EC advised on methods and interpretation of findings.
Funding: The study was supported by a Trauma consortium grant from the Fonds de recherche du Québec—Santé Grant number ‘32543’.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Rubiano AM, Carney N, Chesnut R, et al. . Global neurotrauma research challenges and opportunities. Nature 2015;527:S193–7. 10.1038/nature16035 [DOI] [PubMed] [Google Scholar]
- 2. Corrigan JD, Selassie AW, Orman JA. The epidemiology of traumatic brain injury. J Head Trauma Rehabil 2010;25:72–80. 10.1097/HTR.0b013e3181ccc8b4 [DOI] [PubMed] [Google Scholar]
- 3. Chen A, Bushmeneva K, Zagorski B, et al. . Direct cost associated with acquired brain injury in Ontario. BMC Neurol 2012;12:76 10.1186/1471-2377-12-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tuominen R, Joelsson P, Tenovuo O. Treatment costs and productivity losses caused by traumatic brain injuries. Brain Inj 2012;26:1697–701. 10.3109/02699052.2012.722256 [DOI] [PubMed] [Google Scholar]
- 5. Ciurli P, Formisano R, Bivona U, et al. . Neuropsychiatric disorders in persons with severe traumatic brain injury: prevalence, phenomenology, and relationship with demographic, clinical, and functional features. J Head Trauma Rehabil 2011;26:116–26. [DOI] [PubMed] [Google Scholar]
- 6. van der Naalt J, van Zomeren AH, Sluiter WJ, et al. . Acute behavioural disturbances related to imaging studies and outcome in mild-to-moderate head injury. Brain Inj 2000;14:781–8. 10.1080/026990500421895 [DOI] [PubMed] [Google Scholar]
- 7. Sandel ME, Mysiw WJ. The agitated brain injured patient. Part 1: definitions, differential diagnosis, and assessment. Arch Phys Med Rehabil 1996;77:617–23. 10.1016/S0003-9993(96)90306-8 [DOI] [PubMed] [Google Scholar]
- 8. Kadyan V, Mysiw WJ, Bogner JA, et al. . Gender Differences in Agitation After Traumatic Brain Injury. Am J Phys Med Rehabil 2004;83:747–52. 10.1097/01.PHM.0000140790.30468.F4 [DOI] [PubMed] [Google Scholar]
- 9. Weir N, Doig EJ, Fleming JM, et al. . Objective and behavioural assessment of the emergence from post-traumatic amnesia (PTA). Brain Inj 2006;20:927–35. 10.1080/02699050600832684 [DOI] [PubMed] [Google Scholar]
- 10. Singh R, Venkateshwara G, Nair KPS, et al. . Agitation after traumatic brain injury and predictors of outcome. Brain Inj 2014;28:336–40. 10.3109/02699052.2013.873142 [DOI] [PubMed] [Google Scholar]
- 11. Nott MT, Chapparo C, Baguley IJ. Agitation following traumatic brain injury: An Australian sample. Brain Inj 2006;20:1175–82. 10.1080/02699050601049114 [DOI] [PubMed] [Google Scholar]
- 12. Bogner JA, Corrigan JD, Fugate L, et al. . Role of agitation in prediction of outcomes after traumatic brain injury. Am J Phys Med Rehabil 2001;80:636–44. 10.1097/00002060-200109000-00002 [DOI] [PubMed] [Google Scholar]
- 13. Wolffbrandt MM, Poulsen I, Engberg AW, et al. . Occurrence and severity of agitated behavior after severe traumatic brain injury. Rehabil Nurs 2013;38:133–41. 10.1002/rnj.82 [DOI] [PubMed] [Google Scholar]
- 14. Bogner J, Barrett RS, Hammond FM, et al. . Predictors of agitated behavior during inpatient rehabilitation for traumatic brain injury. Arch Phys Med Rehabil 2015;96:S274–81.e4. 10.1016/j.apmr.2015.04.020 [DOI] [PubMed] [Google Scholar]
- 15. McNett M, Sarver W, Wilczewski P. The prevalence, treatment and outcomes of agitation among patients with brain injury admitted to acute care units. Brain Inj 2012;26:1155–62. 10.3109/02699052.2012.667587 [DOI] [PubMed] [Google Scholar]
- 16. Brooke MM, Questad KA, Patterson DR, et al. . Agitation and restlessness after closed head injury: A prospective study of 100 consecutive admissions. Arch Phys Med Rehabil 1992;73:320–3. 10.1016/0003-9993(92)90003-F [DOI] [PubMed] [Google Scholar]
- 17. Hammond FM, Barrett RS, Shea T, et al. . Psychotropic Medication Use During Inpatient Rehabilitation for Traumatic Brain Injury. Arch Phys Med Rehabil 2015;96:S256–S273.e14. 10.1016/j.apmr.2015.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williamson DR, Frenette AJ, Burry L, et al. . Pharmacological interventions for agitation in patients with traumatic brain injury. Crit Care 2018;22(Suppl 1):P376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoffman AN, Cheng JP, Zafonte RD, et al. . Administration of haloperidol and risperidone after neurobehavioral testing hinders the recovery of traumatic brain injury-induced deficits. Life Sci 2008;83:602–7. 10.1016/j.lfs.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kline AE, Hoffman AN, Cheng JP, et al. . Chronic administration of antipsychotics impede behavioral recovery after experimental traumatic brain injury. Neurosci Lett 2008;448:263–7. 10.1016/j.neulet.2008.10.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kline AE, Massucci JL, Zafonte RD, et al. . Differential effects of single versus multiple administrations of haloperidol and risperidone on functional outcome after experimental brain trauma. Crit Care Med 2007;35:919–24. 10.1097/01.CCM.0000256722.88854.C0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phelps TI, Bondi CO, Ahmed RH, et al. . Divergent long-term consequences of chronic treatment with haloperidol, risperidone, and bromocriptine on traumatic brain injury-induced cognitive deficits. J Neurotrauma 2015;32:590–7. 10.1089/neu.2014.3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fleminger S, Greenwood RRJ, Oliver DL; Cochrane Injuries Group. Pharmacological management for agitation and aggression in people with acquired brain injury. Cochrane Database Syst Rev 2006;73:CD003299 10.1002/14651858.CD003299.pub2 [DOI] [PubMed] [Google Scholar]
- 24. Plantier D, Luauté J, group S. Drugs for behavior disorders after traumatic brain injury: Systematic review and expert consensus leading to French recommendations for good practice. Ann Phys Rehabil Med 2016;59:42–57. 10.1016/j.rehab.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 25. Mehta S, McIntyre A, Janzen S, et al. . Pharmacological management of agitation among individuals with moderate to severe acquired brain injury: A systematic review. Brain Inj 2018;32:287–96. 10.1080/02699052.2017.1419377 [DOI] [PubMed] [Google Scholar]
- 26. Williamson DR, Frenette AJ, Burry L, et al. . Pharmacological interventions for agitation in patients with traumatic brain injury: protocol for a systematic review and meta-analysis. Syst Rev 2016;5:193 10.1186/s13643-016-0374-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 2009;3:e123–30. [PMC free article] [PubMed] [Google Scholar]
- 28. McGowan J, Sampson M, Salzwedel DM, et al. . PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol 2016;75:40–6. 10.1016/j.jclinepi.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 29. Higgins JPT, Altman DG, Gotzsche PC, et al. . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wells G, Shea B, O’Connell D, et al. . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 31. Brooke MM, Patterson DR, Questad KA, et al. . The treatment of agitation during initial hospitalization after traumatic brain injury. Arch Phys Med Rehabil 1992;73:917–21. [PubMed] [Google Scholar]
- 32. Mooney GF, Haas LJ. Effect of methylphenidate on brain injury-related anger. Archives of physical medicine and rehabilitation 1993;74:153–60. [PubMed] [Google Scholar]
- 33. Yablon S, Sherer M, Nakase-Richardson R, et al. . Article 3 (see Poster 14): Amantadine Hydrochloride for Treatment of Symptoms of the Posttraumatic Confusional State: A Randomized, Double-Blind, Placebo-Controlled Trial. Arch Phys Med Rehabil 2010;91:e3 10.1016/j.apmr.2010.07.022 [DOI] [Google Scholar]
- 34. Hammond FM, Sherer M, Malec JF, et al. . Amantadine Effect on Perceptions of Irritability after Traumatic Brain Injury: Results of the Amantadine Irritability Multisite Study. J Neurotrauma 2015;32:1230–8. 10.1089/neu.2014.3803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hammond FM, Bickett AK, Norton JH, et al. . Effectiveness of amantadine hydrochloride in the reduction of chronic traumatic brain injury irritability and aggression. J Head Trauma Rehabil 2014;29:391–9. 10.1097/01.HTR.0000438116.56228.de [DOI] [PubMed] [Google Scholar]
- 36. Gramish JA, Kopp BJ, Patanwala AE. Effect of Amantadine on Agitation in Critically Ill Patients With Traumatic Brain Injury. Clin Neuropharmacol 2017;40:212–6. 10.1097/WNF.0000000000000242 [DOI] [PubMed] [Google Scholar]
- 37. Beresford T, Schmidt BK, Buchanan J, et al. . A double-blind trial of divalproex sodium for affective lability and ethanol use following traumatic brain injurt. APA abstracts 2015. [Google Scholar]
- 38. Maturana Waidele R, Maturana Rodillo R. Control de la agresividad con olanzapina en pacientes post tec / Aggressiveness control using olanzapine in post tbi patients Cienc Trab. 2009;31:22–4. [Google Scholar]
- 39. Baños JH, Novack TA, Brunner R, et al. . Impact of early administration of sertraline on cognitive and behavioral recovery in the first year after moderate to severe traumatic brain injury. J Head Trauma Rehabil 2010;25:357–61. 10.1097/HTR.0b013e3181d6c715 [DOI] [PubMed] [Google Scholar]
- 40. Giacino JT, Whyte J, Bagiella E, et al. . Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med 2012;366:819–26. 10.1056/NEJMoa1102609 [DOI] [PubMed] [Google Scholar]
- 41. Meythaler JM, Depalma L, Devivo MJ, et al. . Sertraline to improve arousal and alertness in severe traumatic brain injury secondary to motor vehicle crashes. Brain Inj 2001;15:321–31. 10.1080/026990501750111274 [DOI] [PubMed] [Google Scholar]
- 42. Schneider WN, Drew-Cates J, Wong TM, et al. . Cognitive and behavioural efficacy of amantadine in acute traumatic brain injury: an initial double-blind placebo-controlled study. Brain Inj 1999;13:863–72. 10.1080/026990599121061 [DOI] [PubMed] [Google Scholar]
- 43. Meythaler JM, Brunner RC, Johnson A, et al. . Amantadine to improve neurorecovery in traumatic brain injury-associated diffuse axonal injury: a pilot double-blind randomized trial. J Head Trauma Rehabil 2002;17:300–13. [DOI] [PubMed] [Google Scholar]
- 44. Tramontana MG, Cowan RL, Zald D, et al. . Traumatic brain injury-related attention deficits: Treatment outcomes with lisdexamfetamine dimesylate (Vyvanse). Brain Inj 2014;28:1461–72. 10.3109/02699052.2014.930179 [DOI] [PubMed] [Google Scholar]
- 45. Fann JR, Bombardier CH, Temkin N, et al. . Sertraline for Major Depression During the Year Following Traumatic Brain Injury: A Randomized Controlled Trial. J Head Trauma Rehabil 2017;32:332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johansson B, Wentzel A-P, Andréll P, et al. . Evaluation of dosage, safety and effects of methylphenidate on post-traumatic brain injury symptoms with a focus on mental fatigue and pain. Brain Inj 2014;28:304–10. 10.3109/02699052.2013.865267 [DOI] [PubMed] [Google Scholar]
- 47. Hart T, Whyte J, Watanabe T, et al. . Effects of dextroamphetamine in subacute traumatic brain injury: A randomized, placebo-controlled pilot study. Journal of neuroscience research 2017. [DOI] [PubMed] [Google Scholar]
- 48. Anderson RL, Birrer KL, DeRyke XL. Haloperidol Use in Acute Traumatic Brain Injury: A Safety Analysis. J Intensive Crit Care 2016;02:1–6. 10.21767/2471-8505.100023 [DOI] [Google Scholar]
- 49. Mysiw WJ, Bogner JA, Corrigan JD, et al. . The impact of acute care medications on rehabilitation outcome after traumatic brain injury. Brain Inj 2006;20:905–11. 10.1080/02699050600743972 [DOI] [PubMed] [Google Scholar]
- 50. Rao N, Jellinek HM, Woolston DC. Agitation in closed head injury: haloperidol effects on rehabilitation outcome. Arch Phys Med Rehabil 1985;66:30–4. [PubMed] [Google Scholar]
- 51. Kooda K, Aho J, Weber D, et al. . 1149. Crit Care Med 2015;43:289 10.1097/01.ccm.0000474980.38145.7b [DOI] [Google Scholar]
- 52. Cummings JL, Mega M, Gray K, et al. . The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–14. 10.1212/WNL.44.12.2308 [DOI] [PubMed] [Google Scholar]
- 53. Greendyke RM, Berkner JP, Webster JC, et al. . Treatment of Behavioral Problems with Pindolol. Psychosomatics 1989;30:161–5. 10.1016/S0033-3182(89)72297-0 [DOI] [PubMed] [Google Scholar]
- 54. Greendyke RM, Kanter DR. Therapeutic effects of pindolol on behavioral disturbances associated with organic brain disease: a double-blind study. J Clin Psychiatry 1986;47:423–6. [PubMed] [Google Scholar]
- 55. Greendyke RM, Kanter DR, Schuster DB, et al. . Propranolol treatment of assaultive patients with organic brain disease. A double-blind crossover, placebo-controlled study. J Nerv Ment Dis 1986;174:290–4. [DOI] [PubMed] [Google Scholar]
- 56. Hammond FM, Malec JF, Zafonte RD, et al. . Potential Impact of Amantadine on Aggression in Chronic Traumatic Brain Injury. J Head Trauma Rehabil 2017;32:308–18. 10.1097/HTR.0000000000000342 [DOI] [PubMed] [Google Scholar]
- 57. Aquired Brain Injury Knowledge Uptake Strategy (ABIKUS) guideline development group. Evidence based recommendations for rehabilitation of moderate to severe acquired brain injury. 2007. http://www.abiebr.com/pdf/abikus_aug_07.pdf.
- 58. Kim E, Bijlani M. A pilot study of quetiapine treatment of aggression due to traumatic brain injury. J Neuropsychiatry Clin Neurosci 2006;18:547–9. 10.1176/jnp.2006.18.4.547 [DOI] [PubMed] [Google Scholar]
- 59. Maryniak O, Manchanda R, Velani A. Methotrimeprazine in the treatment of agitation in acquired brain injury patients. Brain Inj 2001;15:167–74. 10.1080/026990501458399 [DOI] [PubMed] [Google Scholar]
- 60. Stanislav SW, Childs A. Evaluating the usage of droperidol in acutely agitated persons with brain injury. Brain Inj 2000;14:261–5. [DOI] [PubMed] [Google Scholar]
- 61. Wilson MS, Gibson CJ, Hamm RJ. Haloperidol, but not olanzapine, impairs cognitive performance after traumatic brain injury in rats. Am J Phys Med Rehabil 2003;82:871–9. 10.1097/01.PHM.0000091982.33232.CB [DOI] [PubMed] [Google Scholar]
- 62. Ponsford J, Janzen S, McIntyre A, et al. . INCOG recommendations for management of cognition following traumatic brain injury, part I: posttraumatic amnesia/delirium. J Head Trauma Rehabil 2014;29:307–20. [DOI] [PubMed] [Google Scholar]
- 63. Pisa FE, Cosano G, Giangreco M, et al. . Prescribing practice and off-label use of psychotropic medications in post-acute brain injury rehabilitation centres: A cross-sectional survey. Brain Inj 2015;29:508–16. 10.3109/02699052.2014.992474 [DOI] [PubMed] [Google Scholar]
- 64. Fugate LP, Spacek LA, Kresty LA, et al. . Measurement and treatment of agitation following traumatic brain injury: II. a survey of the brain injury special interest group of the american academy of physical medicine and rehabilitation. Arch Phys Med Rehabil 1997;78:924–8. 10.1016/S0003-9993(97)90051-4 [DOI] [PubMed] [Google Scholar]
- 65. Perreault M, Talic J, Frenette A, et al. . Agitation after mild to moderate traumatic brain injury in the intensive care unit. Crit Care 2017;21(Suppl 1):P219. [Google Scholar]
- 66. Chew E, Zafonte RD. Pharmacological management of neurobehavioral disorders following traumatic brain injury--a state-of-the-art review. J Rehabil Res Dev 2009;46:851–79. 10.1682/JRRD.2008.09.0120 [DOI] [PubMed] [Google Scholar]
- 67. Deb S, Crownshaw T. Review of subjectThe role of pharmacotherapy in the management of behaviour disorders in traumatic brain injury patients. Brain Inj 2004;18:1–31. 10.1080/0269905031000110463 [DOI] [PubMed] [Google Scholar]
- 68. Azouvi P, Jokic C, Attal N, et al. . Carbamazepine in agitation and aggressive behaviour following severe closed-head injury: results of an open trial. Brain Inj 1999;13:797–804. 10.1080/026990599121188 [DOI] [PubMed] [Google Scholar]
- 69. Chatham Showalter PE, Kimmel DN. Agitated symptom response to divalproex following acute brain injury. J Neuropsychiatry Clin Neurosci 2000;12:395–7. 10.1176/jnp.12.3.395 [DOI] [PubMed] [Google Scholar]
- 70. Chatham-Showalter PE. Carbamazepine for combativeness in acute traumatic brain injury. J Neuropsychiatry Clin Neurosci 1996;8:96–9. 10.1176/jnp.8.1.96 [DOI] [PubMed] [Google Scholar]
- 71. Wroblewski BA, Joseph AB, Kupfer J, et al. . Effectiveness of valproic acid on destructive and aggressive behaviours in patients with acquired brain injury. Brain Inj 1997;11:37–48. 10.1080/026990597123791 [DOI] [PubMed] [Google Scholar]
- 72. Kim E, Humaran TJ. Divalproex in the management of neuropsychiatric complications of remote acquired brain injury. J Neuropsychiatry Clin Neurosci 2002;14:202–5. 10.1176/jnp.14.2.202 [DOI] [PubMed] [Google Scholar]
- 73. Patel MB, McKenna JW, Alvarez JM, et al. . Decreasing adrenergic or sympathetic hyperactivity after severe traumatic brain injury using propranolol and clonidine (DASH After TBI Study): study protocol for a randomized controlled trial. Trials 2012;13:177 10.1186/1745-6215-13-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Corrigan JD. Development of a scale for assessment of agitation following traumatic brain injury. J Clin Exp Neuropsychol 1989;11:261–77. 10.1080/01688638908400888 [DOI] [PubMed] [Google Scholar]
- 75. Maiuro RD, Vitaliano PP, Cahn TS. A Brief Measure for the Assessment of Anger and Aggression. J Interpers Violence 1987;2:166–78. 10.1177/088626087002002003 [DOI] [Google Scholar]
- 76. Sherer M, Nakase-Thompson R, Yablon SA, et al. . Multidimensional Assessment of Acute Confusion After Traumatic Brain Injury. Arch Phys Med Rehabil 2005;86:896–904. 10.1016/j.apmr.2004.09.029 [DOI] [PubMed] [Google Scholar]
- 77. Keith RA, Granger CV, Hamilton BB, et al. . The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil 1987;1:6–18. [PubMed] [Google Scholar]
- 78. Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry 2007;4:28–37. [PMC free article] [PubMed] [Google Scholar]
- 79. Katz MM, Lyerly SB. Methods for measuring adjustment and social behavior in the community: I. rationale, description, discriminative validity and scale development. Psychol Rep 1963;13:503–35. 10.2466/pr0.1963.13.2.503 [DOI] [Google Scholar]
- 80. Kreutzer J, Seel R, Marwitz J. The Neurobehavioral Functioning Inventory.: The Psychological Corporation. San Antonio, TX, 1999. [Google Scholar]
- 81. Levin HS, High WM, Goethe KE, et al. . The neurobehavioural rating scale: assessment of the behavioural sequelae of head injury by the clinician. J Neurol Neurosurg Psychiatry 1987;50:183–93. 10.1136/jnnp.50.2.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yudofsky SC, Silver JM, Jackson W, et al. . The Overt Aggression Scale for the objective rating of verbal and physical aggression. Am J Psychiatry 1986;143:35–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-029604supp001.pdf (53.9KB, pdf)