Abstract
Introduction
Personalized intervention to increase physical Activity and reduce sedentary behaviour in rehabilitation after Cardiac Operations (PACO) is a smartphone-based and accelerometer-based eHealth intervention to increase physical activity (PA) and reduce sedentary behaviour (SB) among patients recovering from cardiac surgery.
Design
Prospective randomised controlled trial.
Methods and analysis
The present protocol describes a randomised controlled clinical trial to be conducted in the Heart Centres of Kuopio and Turku university hospitals. The trial comprises 540 patients scheduled for elective coronary artery bypass grafting, aortic valve replacement or mitral valve repair. The patients will be randomised into two groups. The control group will receive standard postsurgical rehabilitation guidance. The eHealth intervention group will be given the same guidance together with personalised PA guidance during 90 days after discharge. These patients will receive personalised daily goals to increase PA and reduce SB via the ExSedapplication. Triaxial accelerometers will be exploited to record patients’ daily accumulated PA and SB, and transmit them to the application. Using the accelerometer data, the application will provide online guidance to the patients and feedback of accomplishing their activity goals. The data will also be transmitted to the cloud, where a physiotherapist can monitor individual activity profiles and customise the subsequent PA and SB goals online. The postoperative improvement in patients’ step count, PA, exercise capacity, quality of sleep, laboratory markers, transthoracic echocardiography (TTE) parameters and quality of life, and reduction in SB and incidence of major cardiac events are investigated as outcomes.
Conclusions
The PACO intervention aims to build a personalised eHealth tool for the online tutoring of cardiac surgery patients.
Trial registration number
Keywords: aortic valve replacement, aortic valve stenosis, coronary artery bypass grafting, coronary artery disease, ehealth, exercise, mitral valve regurgitation, mitral valve repair, rehabilitation
Introduction
The ongoing development in diagnostics, drug therapy and invasive procedures has improved the management of cardiovascular diseases (CVDs). However, in spite of this, CVDs still account for a third of all deaths remaining the number one cause of death globally.1 2 Coronary artery disease (CAD), aortic valve stenosis and mitral valve insufficiency are examples of CVDs with high prevalence and mortality.2 The use of specific tools, such as interventional or surgical procedures, are essential in order to relieve patient’s symptoms, improve quality of life (QoL) and reduce mortality, but their costs are remarkable.3 To maximise the benefits of these interventions, there is a need for systematic rehabilitation programmes including optimisation of the patient’s lifestyle (eg, activity profile).4 5
Physical activity (PA) has been found to be useful in the prevention of CVD and also a low-cost and low side effect treatment of CVDs.6–9 The amount of PA before and after cardiac operation has been proven to influence postoperative recovery.5 10 11 However, the beneficial influence of objectively measured PA in postoperative rehabilitation of cardiac surgery patients has been addressed in only few novel studies.5
Sedentary behaviour (SB), in terms of high total time and prolonged bout lengths, is a recently recognised risk factor for cardiometabolic health and CVDs.12 13 On the other hand, there are only limited data on the association between objectively measured long-term SB and the success of rehabilitation after invasive cardiac operations.14 In addition, any reports on the causative impact of reduced SB in the rehabilitation after cardiac procedures do not exist.
In the most recent studies, device-based measurements (eg, accelerometer monitoring) of PA and SB have replaced the questionnaires and self-reports traditionally used in the assessment of patients’ PA profile.14 15 In addition, novel algorithms, such as mean amplitude deviation (MAD) and angle for posture estimation (APE), have improved the analysis of accelerometer data by recognising more specific parameters of PA and SB with good accuracy.16–18 Despite the development in objective measurements, accelerometers have not been exploited in the guidance of increasing PA and reducing prolonged SB bouts in patients recovering from cardiac operations.
The purpose of the present study is to describe the study protocol for ‘Personalized intervention to increase physical Activity and reduce sedentary behaviour in rehabilitation after Cardiac Operations - the PACO trial’. The main objective of the PACO Trial is to evaluate the benefits of smartphone-based and accelerometer -based interactive guidance to increase PA as an eHealth rehabilitation tool for patients recovering from coronary artery bypass grafting (CABG), aortic valve replacement (AVR) and mitral valve repair (MVR) surgery. The results of the trial are expected to facilitate the development of interactive tools and PA guidelines for postoperative rehabilitation of cardiac patients.
Hypothesis
When compared with standard postoperative rehabilitation programmes, a 90-day personalised and interactive rehabilitation guidance (PACO eHealth intervention):
Increases the daily number of steps.
Increases daily PA and decreases daily SB.
Increases the amount of restful sleep.
Improves maximal oxygen consumption (VO2peak).
Improves serum lipids and other laboratory markers.
Improves left ventricular ejection fraction (LVEF) and other TTE parameters.
Improves self-perceived QoL.
Reduces the 12-month incidence of major adverse cardiovascular events among patients undergoing CABG, AVR and MVR procedures.
In addition, we hypothesised that preoperative PA and SB are associated with lower incidence of early postoperative/perioperative complications (eg, postoperative atrial fibrillation, renal failure, prolonged intensive care and acute readmissions) after cardiac surgery.
Methods and analysis
Study population
The PACO Trial will include 540 patients scheduled for elective CABG, AVR or MVR open heart surgery. The needed sample size was calculated with one-sided Mann-Whitney test assuming that the actual distribution is normal. The total sample of 148 (74 in control and 74 in intervention) patients (in each surgery group) is estimated to provide 90% power to detect a difference of 1000 steps between the group means with an estimated group SD of 2000 at a significance level (α) of 0.05. Because of the anticipated loss of about 20% of the patients, 180 patients will be enrolled to each patient group (CABG, AVR and MVR). The mean difference of 1000 steps was estimated on the basis of exercise specialists’ experiences about cardiac surgery patients and pilot patients’ in Kuopio. In addition to pooled analyses, patient groups (CABG, AVR, MVR) will be analysed and evaluated separately, because they have different clinical characteristics (eg, age, comorbidities and risk factors). A patient who meets the following inclusion criteria is eligible for the study: (1) Patient is scheduled for CABG, AVR or/and MVR. (2) Patient is willing to wear a hip/wrist-worn accelerometer. (3) Patient is willing and capable of using a smartphone application during 90 days (is able to use email and/or online bank account independently). Patient will be excluded if: (1) Patient has any severe disease or functional limitation limiting PA (other than CVD). (2) Patient ends up in prolonged intensive care (>2 days in intensive care unit). (3) Patient’s surgery type changes during the operation (eg, surgeon decides to operate tricuspid valve during AVR surgery). (4) Patient has a memory disorder (eg, Alzheimer’s disease). (5) Patient does not use accelerometer as instructed (25% or more of the accelerometer measurement days without accumulated acceleration data). The data will be collected during March 2018–December 2023 (with follow-up to 2028).
Patient and public involvement
Patients were not involved in designing this project.
Design of the intervention
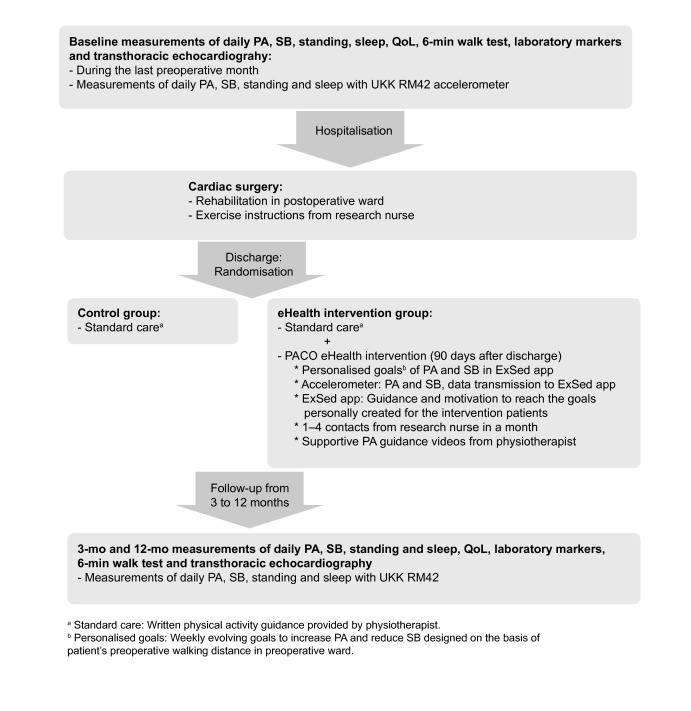
The PACO Trial is a randomised clinical intervention for CABG, AVR and MVR patients conducted during the first 90 days of postoperative rehabilitation after cardiac surgery (figure 1). The baseline PA, SB, standing and sleep profiles will be determined for each patient with 1 week objective accelerometer measurements (UKK RM42, UKK Terveyspalvelut Oy, Tampere, Finland) during the last preoperative month.16–19 In addition, baseline laboratory markers, VO2 and TTE (TTE: the patients of Kuopio University Hospital) will be assessed in the preoperative ward before the procedure.
Figure 1.
The implementation of the PACO Trial. PA, physical activity; QoL, quality of life; SB, sedentary behaviour.
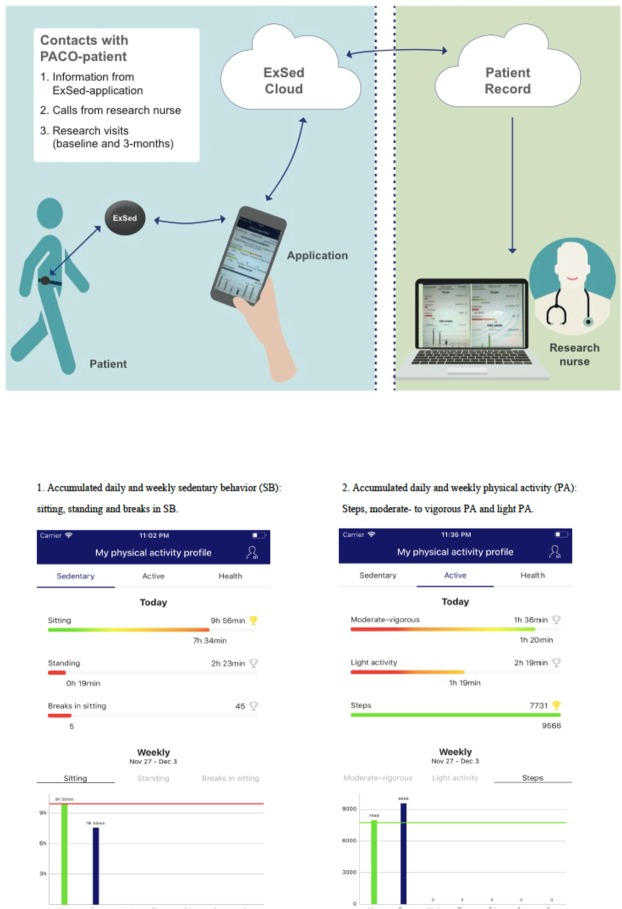
After the baseline measurements, the patients will be randomly assigned into two groups: eHealth intervention combined with standard care (intervention group) or standard care (control group). Randomisation will be executed by a statistician with a randomised block design using centre (Kuopio vs Turku), gender and type of surgery (CABG, AVR, MVR, CABG+AVR, CABG+MVR) as blocks. At the randomisation stage the statistician is blinded from all patient information except centre, gender and type of surgery. After the surgery, the patients of both control and eHealth intervention groups will undergo a standard postoperative rehabilitation programme. According to the normal practice of the participating hospitals an oral and written rehabilitation guidance including PA guidance, and exercise training instructions will be provided to all the patients by a physiotherapist in the postoperative ward. Besides the standard programme, the patients of the eHealth intervention group will receive an accelerometer containing specific algorithms for PA and SB (ExSed Movesense, Suunto, Espoo, Finland) together with a smartphone application (ExSed, UKK Terveyspalvelut Oy, Tampere, Finland). Research smartphones will be provided to the intervention patients by the research group. The ExSed application is intended to be used during the first 90 days of their postoperative rehabilitation after discharge. The application communicates with a dedicated cloud system. Weekly evolving, daily goals to increase PA and reduce SB bouts will be devised personally for each patient in the intervention group. A new goal will be sent to the patient’s ExSed application from the cloud system at the beginning of each intervention week. The initial step count goal of the first postdischarge week will be personally calculated as approximately 3 × the walking distance in a 6-min walk test assessed in the preoperative ward. After the first postdischarge week, the patient’s subsequent weekly goals with evolving step counts are personally defined as about 10%–15% higher than the recorded step count of the preceding week. The step counts will be increased progressively so that the target of each eHealth intervention patient is at least 10 000 steps daily at the end of the intervention. The cloud system allows a research nurse to monitor the accomplishment of these goals, and if necessary, modify the forthcoming goals online to make them more suitable for each patient (figure 2).
Figure 2.
The contacts with the eHealth intervention patient and the view of the ExSed application for eHealth intervention patients.
The ExSed Movesense accelerometer will record and store all data of accumulated PA, SB, standing and sleep (24 hours/day) in 6 s epochs using the same algorithms as the UKK RM42 accelerometer used in the preoperative measurements. In addition to this, the patient will receive interactive feedback on accumulated PA, SB, standing and sleep via the ExSed application. Using the information of daily accumulated PA and SB, the ExSed application is expected to guide and motivate the intervention patients to accomplish their personalised goals (number of steps) each day. Personal guidance and feedback are transmitted as illustrative histograms (graphical columns illustrate patient’s activity and achievement of their daily goals) and motivational pop-up messages (figure 2). With these arrangements, the eHealth intervention patients can observe online the accumulation and sufficiency of their daily activity by monitoring the ExSed application whenever most convenient for them. The feedback is online, that is, a patient can monitor their daily step count and activity goal. Therefore, the time and frequency of personalised feedback depends on the patient’s own activity with their smartphone. The patients are encouraged to open the application at least once daily, preferably more often. Short pop-up messages, informing the patient about their prolonged sitting time, will appear on a locked screen if the patient has been sitting still for longer than 60 min.
In order to ensure that the patient is motivated, the device is operating and the intervention is proceeding as intended, the eHealth intervention patients will be contacted with a structured contact form once to four times monthly by a study nurse via mobile phone and provided with supplementary video material via the ExSed application (see below). The intervention patients with a sedentary activity profile (not meeting their PA goals) according to the data in the cloud system will especially be contacted to enhance their commitment to the intervention. During the conversations, the study nurse will also ask about health issues that could limit the patient’s PA and help them overcome possible barriers. The activity goals are automatically updated to the patient’s smartphone.
A follow-up of PA, SB, standing and sleep profiles will be conducted to the intervention group with the Movesense accelerometer and to the control group with the UKK RM42 accelerometer 3 months postdischarge, and to all patients with the UKK RM42 accelerometer 12 months postdischarge. In addition, self-perceived QoL, incidence of CVD events, laboratory markers, TTE and heart rate variability will be assessed after 3-month and 12 month follow-ups.
Supplementary video files
Besides the personalised activity goals and interactive guidance, supplementary videos containing exercise training instructions (eg, instructions for stretching) and PA guidance (eg, instructions to use stairs instead of an elevator) are provided to the eHealth intervention patients to increase their postoperative PA and to reduce prolonged SB bouts. The videos include five short files (lasting few minutes) to motivate the patients to be physically active, supporting the interactive guidance given by the ExSed application. The content of each video depends on the time (weeks) from the surgery and the postoperative functional capability (daily steps) of the patient. If the patient is performing relatively well after the cardiac operation, the first videos with lowest levels of activity guidance can be bypassed and the patient can move straight to the more physically challenging exercises.
Accelerometer recordings
Tracking of patient’s PA, SB, standing and sleep will be conducted with hip-worn and wrist-worn triaxial UKK RM42 and Movesense accelerometers. Both accelerometers will be used at the hip when patients are awake and at the wrist during sleep. The UKK RM42 accelerometer will be used in all patients preoperatively and 12 months postoperatively, and in the control group during the postoperative 3-month follow-up. The raw data from UKK RM42 will be collected to the hard disk for further analysis without any patient feedback whereas the Movesense accelerometer used in the eHealth intervention group transfers the preprocessed data in 6 s epochs via the smartphone to a cloud system for analysis. Analysed information will be transferred back to the smartphone and will be shown to the patient with the interactive ExSed application. The research patients will receive written and oral guidance about using the accelerometers properly.
Analysis of the accelerometer data
The analysis of the triaxial raw acceleration data is based on novel validated MAD and APE (MAD-APE) algorithms.16–18 MAD represents values of the resultant acceleration of the three orthogonal acceleration components and will be determined in 6 s epochs according to our standard procedures. MAD values have been found to be valid indicators of incident energy consumption during locomotion.16 17 As recently recommended, MAD values will be converted to metabolic equivalents (MET=3.5 mL/kg/min of oxygen consumption) for each epoch.20
Patients’ mean daily number of steps and PA will be recorded, and PA will be classified into three categories in terms of METs: light PA (1.5–2.9 MET), moderate PA (MPA=3.0–6.0 MET) and vigorous PA (VPA >6.0 METs).21 In addition, MPA and VPA can be combined into moderate-to vigorous PA (MVPA=3.0 or more METs).
SB is defined as time spent in seated or reclined position with less than 1.5 MET energy consumption. Standing still is not included in SB, but it will be analysed separately.22 The classification of the body posture into lying, sitting and standing is based on the APE algorithm in combination with analysis of MAD, which has shown about 90% accuracy in free-living conditions also.18 In addition, the number of breaks in SB (SB bout ending with vertical acceleration and change in body posture) will be assessed.
Sleep characterised by accelerometer primarily represents a sustained lack of wrist movement when the participant reports being in bed.23 The sleep algorithm monitors the change in wrist orientation between consecutive epochs and calculates the time interval between changes that exceed five degrees. The sleep is classified into three categories (awake, restless and restful) according to the abovementioned time intervals between the changes.
Because the acceleration data are collected continuously during the 24 hours circadian cycle, several parameters describing patients’ daily PA, SB, standing and sleep profiles can be assessed. For PA, SB and standing, the total times, and number and accumulated times of the following bout lengths (eg, >30 s, >1 min, >3 min, >15 min) will be analysed. In addition, the mean and maximum numbers of daily steps will be calculated for each patient. The daily mean and peak MET levels will also be determined.12
Primary outcomes
The primary outcome variable of the trial is the change in mean daily step count between the baseline (preoperatively) and at 3 months from hospital discharge. The mean daily number of steps will be assessed with 7-day accelerometer recordings of PA for each patient at baseline and after 3 months of rehabilitation as well as 12 months postoperatively.
Secondary outcomes
The secondary outcomes of the trial are:
Increase in mean daily step count between baseline and 12 months from hospital discharge.
Increase in the mean daily total time and number of light PA and MVPA bouts between baseline and 3 (and 12) months from hospital discharge.
Decrease in mean daily total time and number of SB bouts between baseline and 3 (and 12) months from hospital discharge.
Increase in the portion of restful sleep between baseline and 3 (and 12) months from hospital discharge.
Improvement in VO2 peak between baseline and 3 months from hospital discharge.
Improvement in self-perceived QoL (QoL; The 15D, Patient Health Questionnaire 2, Seattle Angina Questionnaire 7+, Rose Dyspnea Scale and EuroQoL-5D) between baseline and 3 (and 12) months from hospital discharge.24 25
Improvement in LVEF and other TTE parameters between baseline and 3 months from hospital discharge.
Decrease in the incidence of major cardiovascular events assessed after 12 postsurgical months.
Improvement in the laboratory markers between baseline and 3 months from discharge.
Improvement in heart rate variability between the baseline and 3 months from discharge among patients undergoing PACO eHealth programme as a part of their postsurgical rehabilitation.
Maximal oxygen consumption
A 6-min walk test will be conducted for all patients in the preoperative ward at baseline and after 3 months of postoperative rehabilitation and 12 months after surgery according to the guidelines of the American Thoracic Society (ATS).26 The VO2 peak will be calculated for the patients according to the ATS statement.
Major cardiovascular events
The major cardiovascular events include the following postoperative events: all-cause mortality, any rehospitalisations due to CVD (including acute readmissions), repeat coronary revascularisation, non-fatal myocardial infarction and stroke. The incidence of CVD events will be recorded from the patient records and from the national registry of hospital admissions and discharge diagnoses maintained by the National Institute for Health and Welfare. Deaths and causes of death will be recorded from the patient records and from Statistics Finland a national registry of deceased patients including the causes of death.
Laboratory markers
Laboratory markers: Low-density lipoprotein (LDL) cholesterol, oxidised LDL cholesterol, High-density lipoprotein (HDL) cholesterol, oxidised HDL cholesterol, total cholesterol, triglycerides, haemoglobin, leucocytes, thrombocytes, glucose, glycated haemoglobin (HbA1c), potassium, creatinine, pro-brain natriuretic peptide (proBNP) will determined in participating hospitals in the preoperative ward at baseline, and 3 months and 12 months after discharge. In addition, 5 mL of plasma and 3 mL of serum will be sent to the freezer for further analysis of inflammatory markers (eg, interleukins).
Transthoracic echocardiography
Transthoracic echocardiography (TTE) will be performed at baseline and 3 months and 12 months after the operation. For measurement of LVEF, the modified biplane Simpson’s rule will be used. Left Ventricular (LV) end-diastolic volume and LV end-systolic volume are obtained from the apical four chambers and two chambers.27 Transmitral inflow includes the peak early filling (E wave) and late diastolic filling (A wave) velocities. The E/A ratio, deceleration time of the E wave and E velocity divided by mitral annular E’ velocity (E/E’) are used to assess left ventricular diastolic function. Right ventricular systolic function will be assessed using tricuspid annular plane systolic excursion (TAPSE).
Statistical analysis plan
Between-group differences from baseline to 3 months and baseline to 12 months will be analysed with analysis of covariance or generalised linear models depending on the distribution of the outcome measure. Additionally, the data will be analysed longitudinally using three time points (baseline; 3 months and 12 months) with generalised linear mixed models.
Ethics and dissemination
The trial has been registered with clinicaltrials.gov register (NCT03470246). The research will be conducted by following the guidelines of good scientific practice and provisions of the Finnish Medical Research Act (Declaration of Helsinki). All the study participants will sign a written informed consent before participation.
The results will be published in 10–15 articles in high-level journals of cardiology, cardiothoracic surgery, general medicine and exercise medicine.
Discussion
The PACO Trial is a pragmatic prospective randomised controlled clinical intervention, designed and conducted in collaboration with two university hospitals in Finland. As far as we know, the trial will be the first one investigating the effectiveness of personalised interactive accelerometer-based eHealth guidance to increase PA and reduce SB in the postoperative rehabilitation of patients recovering from open heart surgery. In addition, as secondary end points we explore whether the eHealth intervention improves patients’ physical capacity and QoL, and reduces adverse cardiovascular events. For the first time, patients undergoing elective cardiac procedures will receive evolving personalised activity goals on a weekly basis as well as online accelerometer-based feedback of their PA to their personal smartphone. There is a lack of device-based evidence indicating the virtual effects of online PA tutoring on the patients’ recovery after cardiac procedures.
As recently suggested in a position paper of the European Association of Preventive Cardiology, exercise training should be promoted among patients with CVD and especially type 2 diabetes mellitus to enhance the patient’s lipid profile, exercise capacity and other cardiovascular functions.28 There is solid evidence indicating that physical exercise as a part of the rehabilitation programme increases PA and improves exercise capacity in patients with CAD and other CVDs.9 29 30 The most encouraging results suggest that exercise interventions could be effective tools in reducing the incidence of CVD rehospitalisations and even mortality among the patients with CAD.9 31 32 Exercise intervention has also been reported to improve cardiorespiratory fitness in patients recovering from valve surgery.33–35 However, in these studies training was conducted in a laboratory environment with a bicycle ergometer or other aerobic exercises supervised by healthcare personnel. Thus, implementation of these supervised face-to-face PA interventions requires remarkable amount of labour and financial support as well as patient’s time, which induces a great challenge for both public healthcare and the patient. It would be of benefit if the training programme could be employed at home or at work as a part of daily life.
So far, there is no definite evidence that home-based online PA interventions during rehabilitation from cardiac surgery can improve the patient’s QoL, functional capability and especially, prognosis.36 37 However, some previous prospective studies have found online interventions potentially beneficial in improving patients’ VO2 peak and weight loss, and preventing rehospitalisations during the postoperative rehabilitation of cardiac patients.4 5 If the effectiveness of online PA guidance will be further improved by using personalised training goals and interactive feedback, the rehabilitation programme could be strengthened and the cost-effectiveness of invasive CVD therapy might be developed.3 38 In addition, instead of focusing only on PA, the causative influence of replacing SB bouts with standing and light PA has not been investigated among patients with a cardiac procedure.
In the PACO Trial, objective measurements of PA and SB will be conducted with triaxial accelerometers. As previously reported, accelerometers are more sensitive tools for assessing patients’ activity and for revealing associations between PA and SB with CVD biomarkers than self-reports.15 21 However, objective measurements have rarely been adapted in postoperative cardiac patients.14 In the PACO Trial the accelerometer data will be analysed with novel, validated MAD and APE algorithms.16–18 The algorithms have been exploited in our recent studies investigating objectively measured PA and SB in high CVD risk subjects.12 39 By means of these algorithms, we are able to determine more specific parameters of PA and SB than the commonly used total times, such as numbers and accumulated times of different PA and SB bout lengths (eg, 1 min, 30 min), and use them to improve guidance for patients. In addition, breaks and different postures in SB can be evaluated.18 Previous studies have most commonly applied proprietary count-based algorithms in the analysis of raw acceleration data. However, because of the variations in cut points and algorithms, count-based data are not comparable between various devices used in previous studies. The MAD-APE algorithms are universal and applicable in all devices collecting triaxial acceleration data in raw mode and thus provide comparable results irrespective of accelerometer brands.16
Due to the device-based nature of PA and SB measurements, the change in mean daily step count represents objective information about the effectiveness of the PACO eHealth intervention. The daily step count is also practical and easy to understand for all the patients, which is why it is used in providing goals and activity guidance in the ExSed application. In addition, according to previous literature, we hypothesised that the influence of the PACO eHealth intervention might be strongest, when it comes to patients’ habitual activity.36 37 Therefore, the change in mean daily step count was selected to be the primary outcome parameter of the PACO Trial. Return to work was not included in secondary outcomes due to the fact that, in Finland, significant proportion of cardiac surgery patients are pensioners.
TTE parameters, representing cardiac systolic (eg, LVEF, TAPSE) and diastolic (eg, E/A ratio, E/E’) functions are used as secondary outcomes of this trial due to the previous findings of the influence of exercise training on cardiac function.40 There is no definite information on the specific mechanisms of the influence of exercise training on cardiac function. However, it has been noted that exercise training decreases heart rate and blood pressure at rest, which could lead to decreased oxygen consumption in the myocardium and further improved cardiac systolic and diastolic functions.41
There is evidence that low preoperative PA is associated with higher incidence of perioperative and postoperative complications.10 One potential hypothesis is that good cardiorespiratory fitness speeds up recovery from cardiopulmonary bypass. Therefore, recovery from operation and complications including acute and late readmissions will be addressed in the PACO Trial.
Associations between high habitual PA and improved plasma biomarkers (increased HDL cholesterol, and decreased total cholesterol, triglycerides, glucose and HbA1c) have been stated in populations with different CVD risk profiles.42 It has also been noted that exercise training is able to reduce oxidised LDL cholesterol.43 In addition, high PA is stated to be associated with lower concentrations of cardiac biomarkers (proBNP) in a population-based sample.44 However, there is little information on the influence of PA interventions on plasma biomarkers among cardiac surgery patients. Therefore, we included these biomarkers to secondary outcomes of the PACO Trial.
In addition to exercise interventions, individualised interventions and telephone follow-ups have been used to facilitate the rehabilitation process after cardiac surgery. For example, interhospital cooperation has been used to improve postoperative QoL and activities of daily living among cardiac surgery patients with promising results.45 Furthermore, telemedicine interventions have been found to be beneficial in improving patients’ exercise capacity.46
The PACO Trial has several strengths of which the inclusive samples of cardiac surgery patients is one of the major one. Diverse markers representing the outcomes of the intervention, objective measurements of patients’ PA and SB, and state-of-the-art analysis algorithms for accelerometer data are also major assets of the present study. However, the most essential novelty of the PACO Trial is the eHealth intervention based on an innovative combination of accelerometer, interactive smartphone application and cloud system, which has not previously been adapted to the rehabilitation programme of patients with a cardiac procedure.
Limitations
The PACO Trial has limitations also. The fact that baseline PA and SB may differ from patients’ normal habitual activity profiles, because they are scheduled for major cardiac operation, is one of them. This might overstate the change between preoperative and postoperative step counts. The operation type may change during the surgery resulting in patient exclusion. Furthermore, many patients have comorbities (eg, respiratory infections) during their postoperative rehabilitation, which will affect their ability to constantly reach their daily step count goals. In addition, seasonal changes in weather influence patients’ ability to exercise outdoors, which affects their daily steps. In addition, unlike Movesense, the UKK RM42 accelerometer is not supposed to be used in water, which might lead to loss of a small proportion of PA data.
Conclusions
In order to improve patient’s postoperative recovery, PA interventions should be more feasible than the standard postoperative methods today and they should be exploited in the rehabilitation of cardiac surgery. However, it is challenging to incorporate supervised PA interventions in clinical settings, which is why online PA guidance should be effectively developed. The PACO eHealth intervention introduces a novel tool for postoperative activity guidance of cardiac surgery patients. Accurate objective measurement of PA and SB, daily evolving goals to replace PA and SB, and the motivating feedback for fulfilling them will be provided to the patients using a combination of smartphone application and accelerometer. A novel cloud system will be used to monitor their activity profiles and personalise the goals more suitable for each patient. As far as we know, the PACO Trial is the first one investigating the effectiveness of personalised interactive eHealth guidance on improving PA, cardiorespiratory fitness, laboratory markers and TTE parameters, and reducing SB and cardiovascular outcomes in the postoperative rehabilitation process of cardiac surgery patients.
Acknowledgments
The authors thank Tarja Tuomainen, Teemu Haapala and Jyry Kuukkanen for their support to this study.
Footnotes
Contributors: VV, JH, PH, HV-Y, KT, JS, HS, VA, JA, TV and JHar contributed to the conception or design of the work. VV, JH, KT, TV and JHar contributed to the acquisition, analysis or interpretation of the data for the work. VV drafted the manuscript. All authors critically revised the manuscript and gave final approval and agreed to be accountable for all aspects of the work ensuring integrity and accuracy.
Funding: This work was supported by Yrjö Jahnsson Foundation (grant number 6992), Research Foundation of North Savo Hospital District (VTR), and Ministry of Education and Culture of Finland.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The Ethics committee of North Savo Hospital district (ID: 304/2017).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: There are no data in this work. All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Wang H, Abajobir AA, Abate KH, et al. . Global, regional, and national under-5, adult mortality, age-specific mortality and life-expectancy, 1970-2016: a systematic analysis for global burden of Disease Study. Lancet 2017;270:1084–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth GA, Johnson C, Abajobir A, et al. . Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 2017;70:1–25. 10.1016/j.jacc.2017.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vieira Ricardo D'Oliveira, Hueb W, Hlatky M, et al. . Cost-effectiveness analysis for surgical, angioplasty, or medical therapeutics for coronary artery disease: 5-year follow-up of medicine, angioplasty, or surgery Study (MASS) II trial. Circulation 2012;126(11 Suppl 1):S145–S150. 10.1161/CIRCULATIONAHA.111.084442 [DOI] [PubMed] [Google Scholar]
- 4.Coorey GM, Neubeck L, Mulley J, et al. . Effectiveness, acceptability and usefulness of mobile applications for cardiovascular disease self-management: systematic review with meta-synthesis of quantitative and qualitative data. Eur J Prev Cardiol 2018;25:505–21. 10.1177/2047487317750913 [DOI] [PubMed] [Google Scholar]
- 5.Frederix I, Driessche NV, Hansen D, et al. . Increasing the medium-term clinical benefits of hospital-based cardiac rehabilitation by physical activity telemonitoring in coronary artery disease patients. Eur J Prev Cardiol 2015;22:150–8. 10.1177/2047487313514018 [DOI] [PubMed] [Google Scholar]
- 6.Ekblom-Bak E, Ekblom B, Vikström M, et al. . The importance of non-exercise physical activity for cardiovascular health and longevity. Br J Sports Med 2014;48:233–8. 10.1136/bjsports-2012-092038 [DOI] [PubMed] [Google Scholar]
- 7.Lear SA, Hu W, Rangarajan S, et al. . The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. The Lancet 2017;390:2643–54. 10.1016/S0140-6736(17)31634-3 [DOI] [PubMed] [Google Scholar]
- 8.Mann S, Jimenez A, Domone S, et al. . Comparative effects of three 48-week community-based physical activity and exercise interventions on aerobic capacity, total cholesterol and mean arterial blood pressure. BMJ Open Sport Exerc Med 2016;2:e000105 10.1136/bmjsem-2015-000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson L, Oldridge N, Thompson DR, et al. . Exercise-Based cardiac rehabilitation for coronary heart disease: cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016;67:1–12. 10.1016/j.jacc.2015.10.044 [DOI] [PubMed] [Google Scholar]
- 10.van Laar C, TImman ST, Noyez L. Decreased physical activity is a predictor for a complicated recovery post cardiac surgery. Health Qual Life Outcomes 2017;15 10.1186/s12955-016-0576-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi T, Kumamaru M, Jenkins S, et al. . In-patient step count predicts re-hospitalization after cardiac surgery. J Cardiol 2015;66:286–91. 10.1016/j.jjcc.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 12.Vasankari V, Husu P, Vähä-Ypyä H, et al. . Association of objectively measured sedentary behaviour and physical activity with cardiovascular disease risk. Eur J Prev Cardiol 2017;24:1311–8. 10.1177/2047487317711048 [DOI] [PubMed] [Google Scholar]
- 13.Chau JY, Grunseit A, Midthjell K, et al. . Sedentary behaviour and risk of mortality from all-causes and cardiometabolic diseases in adults: evidence from the HUNT3 population cohort. Br J Sports Med 2015;49:737–42. 10.1136/bjsports-2012-091974 [DOI] [PubMed] [Google Scholar]
- 14.Prince SA, Blanchard CM, Grace SL, et al. . Objectively-measured sedentary time and its association with markers of cardiometabolic health and fitness among cardiac rehabilitation graduates. Eur J Prev Cardiol 2016;23:818–25. 10.1177/2047487315617101 [DOI] [PubMed] [Google Scholar]
- 15.Celis-Morales CA, Perez-Bravo F, Ibañez L, et al. . Objective vs. self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PLoS One 2012;7:e36345 10.1371/journal.pone.0036345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vähä-Ypyä H, Vasankari T, Husu P, et al. . Validation of Cut-Points for evaluating the intensity of physical activity with Accelerometry-Based mean amplitude deviation (Mad). PLoS One 2015;10:e0134813 10.1371/journal.pone.0134813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vähä-Ypyä H, Vasankari T, Husu P, et al. . A universal, accurate intensity-based classification of different physical activities using RAW data of accelerometer. Clin Physiol Funct Imaging 2015;35:64–70. 10.1111/cpf.12127 [DOI] [PubMed] [Google Scholar]
- 18.Vähä-Ypyä H, Husu P, Suni J, et al. . Reliable recognition of lying, sitting, and standing with a hip-worn accelerometer. Scand J Med Sci Sports 2018;28:1092–102. 10.1111/sms.13017 [DOI] [PubMed] [Google Scholar]
- 19.Ng KW, Rintala P, Husu P, et al. . Device-based physical activity levels among Finnish adolescents with functional limitations. Disabil Health J 2019;12:114–20. 10.1016/j.dhjo.2018.08.011 [DOI] [PubMed] [Google Scholar]
- 20.Sievänen H, Kujala UM. Accelerometry-Simple, but challenging. Scand J Med Sci Sports 2017;27:574–8. 10.1111/sms.12887 [DOI] [PubMed] [Google Scholar]
- 21.Strath SJ, Kaminsky LA, Ainsworth BE, et al. . Guide to the assessment of physical activity: clinical and research applications: a scientific statement from the American Heart Association. Circulation 2013;128:2259–79. 10.1161/01.cir.0000435708.67487.da [DOI] [PubMed] [Google Scholar]
- 22.Tremblay MS, Aubert S, Barnes JD, et al. . Sedentary behavior Research network (SBRN) – terminology consensus project process and outcome. Int J Behav Nutr Phys Act 2017;14 10.1186/s12966-017-0525-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Hees VT, Sabia S, Anderson KN, et al. . A novel, open access method to assess sleep duration using a Wrist-Worn Accelerometer. PLoS One 2015;10:e0142533 10.1371/journal.pone.0142533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Ann Med 2001;33:328–36. 10.3109/07853890109002086 [DOI] [PubMed] [Google Scholar]
- 25.Chan PS, Jones PG, Arnold SA, et al. . Development and validation of a short version of the Seattle angina questionnaire. Circ Cardiovasc Qual Outcomes 2014;7:640–7. 10.1161/CIRCOUTCOMES.114.000967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories Ats statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 27.Lang RM, Badano LP, Mor-Avi V, et al. . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–71. 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 28.Kemps H, Kränkel N, Dörr M, et al. . Exercise training for patients with type 2 diabetes and cardiovascular disease: what to pursue and how to do it. A position paper of the European association of preventive cardiology (EAPC). Eur J Prev Cardiol 2019;26:709–27. 10.1177/2047487318820420 [DOI] [PubMed] [Google Scholar]
- 29.Saberi S, Wheeler M, Bragg-Gresham J, et al. . Effect of moderate-intensity exercise training on peak oxygen consumption in patients with hypertrophic cardiomyopathy: a randomized clinical trial. JAMA 2017;317:1349–57. 10.1001/jama.2017.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belardinelli R, Paolini I, Cianci G, et al. . Exercise training intervention after coronary angioplasty: the ETICA trial. J Am Coll Cardiol 2001;37:1891–900. 10.1016/S0735-1097(01)01236-0 [DOI] [PubMed] [Google Scholar]
- 31.Lee J-Y, Ahn J-M, Park D-W, et al. . Impact of exercise-based cardiac rehabilitation on long-term clinical outcomes in patients with left main coronary artery stenosis. Eur J Prev Cardiol 2016;23:1804–13. 10.1177/2047487316658570 [DOI] [PubMed] [Google Scholar]
- 32.Hambrecht R, Walther C, Möbius-Winkler S, et al. . Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation 2004;109:1371–8. 10.1161/01.CIR.0000121360.31954.1F [DOI] [PubMed] [Google Scholar]
- 33.Sibilitz KL, Berg SK, Tang LH, et al. . Exercise-based cardiac rehabilitation for adults after heart valve surgery. Cochrane Database Syst Rev 2016;3:CD010876 10.1002/14651858.CD010876.pub2 [DOI] [PubMed] [Google Scholar]
- 34.Lin CY, He Z, Chen J, et al. . Efficacy analysis of rehabilitation therapy on patients with heart valve replacement. Chinese Journal of Clinical Rehabilitation 2004;8:426–7. [Google Scholar]
- 35.Sire S. Physical training and occupational rehabilitation after aortic valve replacement. Eur Heart J 1987;8:1215–20. 10.1093/oxfordjournals.eurheartj.a062195 [DOI] [PubMed] [Google Scholar]
- 36.Maddison R, Pfaeffli L, Whittaker R, et al. . A mobile phone intervention increases physical activity in people with cardiovascular disease: results from the heart randomized controlled trial. Eur J Prev Cardiol 2015;22:701–9. 10.1177/2047487314535076 [DOI] [PubMed] [Google Scholar]
- 37.Reid RD, Morrin LI, Beaton LJ, et al. . Randomized trial of an Internet-based computer-tailored expert system for physical activity in patients with heart disease. Eur J Prev Cardiol 2012;19:1357–64. 10.1177/1741826711422988 [DOI] [PubMed] [Google Scholar]
- 38.Frederix I, Hansen D, Coninx K, et al. . Effect of comprehensive cardiac telerehabilitation on one-year cardiovascular rehospitalization rate, medical costs and quality of life: a cost-effectiveness analysis. Eur J Prev Cardiol 2016;23:674–82. 10.1177/2047487315602257 [DOI] [PubMed] [Google Scholar]
- 39.Vasankari V, Husu P, Vähä-Ypyä H, et al. . Subjects with cardiovascular disease or high disease risk are more sedentary and less active than their healthy Peers. BMJ Open Sport Exerc Med 2018;4:e000363 10.1136/bmjsem-2018-000363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu L, Cai Z, Xiong M, et al. . Efficacy of an early home-based cardiac rehabilitation program for patients after acute myocardial infarction: a three-dimensional speckle tracking echocardiography randomized trial. Medicine 2016;95:e5638 10.1097/MD.0000000000005638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varghese T, Schultz WM, McCue AA, et al. . Physical activity in the prevention of coronary heart disease: implications for the clinician. Heart 2016;102:904–9. 10.1136/heartjnl-2015-308773 [DOI] [PubMed] [Google Scholar]
- 42.Qi Q, Strizich G, Merchant G, et al. . Objectively measured sedentary time and cardiometabolic biomarkers in US Hispanic/Latino adults: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Circulation 2015;132:1560–9. 10.1161/CIRCULATIONAHA.115.016938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasankari TJ, Kujala UM, Vasankari TM, et al. . Reduced oxidized LDL levels after a 10-month exercise program. Med Sci Sports Exerc 1998;30:1496–501. 10.1097/00005768-199810000-00005 [DOI] [PubMed] [Google Scholar]
- 44.Parsons TJ, Sartini C, Welsh P, et al. . Objectively measured physical activity and cardiac biomarkers: a cross sectional population based study in older men. Int J Cardiol 2018;254:322–7. 10.1016/j.ijcard.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morisawa T, Ueno K, Fukuda Y, et al. . Significance of sequential cardiac rehabilitation program through inter-hospital cooperation between acute care and rehabilitation hospitals in elderly patients after cardiac surgery in Japan. Heart Vessels 2017;32:1220–6. 10.1007/s00380-017-0983-6 [DOI] [PubMed] [Google Scholar]
- 46.Scalvini S, Zanelli E, Comini L, et al. . Home-based exercise rehabilitation with telemedicine following cardiac surgery. J Telemed Telecare 2009;15:297–301. 10.1258/jtt.2009.090208 [DOI] [PubMed] [Google Scholar]