Fig. 3.
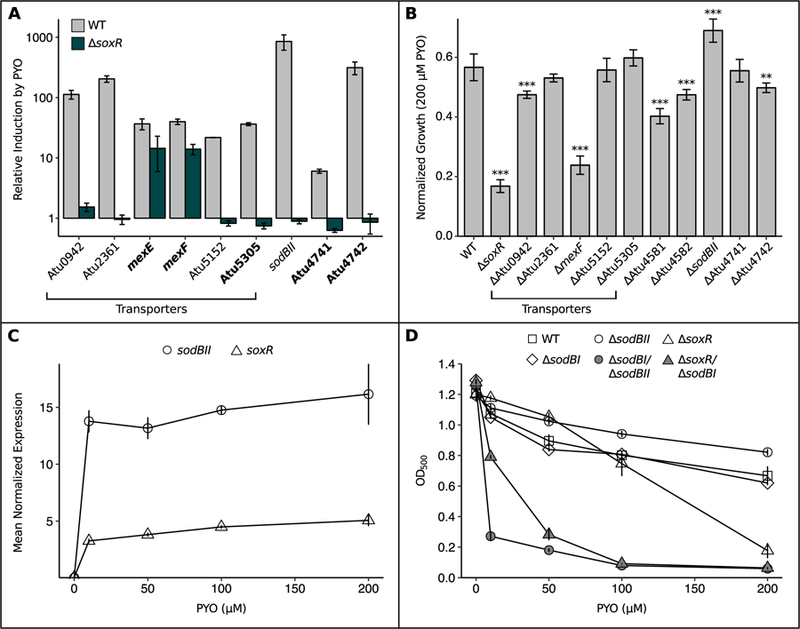
SoxR protects A. tumefaciens against PYO by upregulating functionally redundant superoxide dismutase, transporters and redox-related genes.
A) qRT-PCR validation of putative members of the SoxR regulon, showing that induction of these genes upon a 20 min exposure to 100 µM PYO was partially or fully abrogated in the ∆SoxR mutant (n = 3). Bolded genes lack a SoxR box and thus could not be identified as SoxR-regulated by computational approaches in earlier studies. qRT-PCR validation was not performed for Atu4581 and Atu4582, as these genes are predicted to be co-transcribed with sodBII (Mao et al., 2009). Expression levels were normalized to the housekeeping gene rpoD, and induction was calculated as the expression in the presence of PYO divided by the expression without PYO. B) Growth of single knockout mutants for members of the SoxR regulon after 24 hrs in the presence of 200 µM PYO, normalized to growth of parallel cultures without PYO. ** p < 0.01, *** p < 0.001 (n = 3, linear regression with dummy variable coding using WT as the reference group). C) Normalized expression levels of sodBII and soxR in WT after 20 min exposure to different concentrations of PYO (n = 3). Expression levels were determined by qRT-PCR and normalized to rpoD. D) Growth of WT, ∆sodBI, ∆sodBII, ∆soxR, and the ∆sodBI/∆sodBII and ∆soxR/∆sodBI mutants after 24 hrs in the presence of different concentrations of PYO (n ≥ 3). Error bars in all panels represent standard deviations of biological replicates. In B and D, cultures were in stationary phase at the reported time point.
