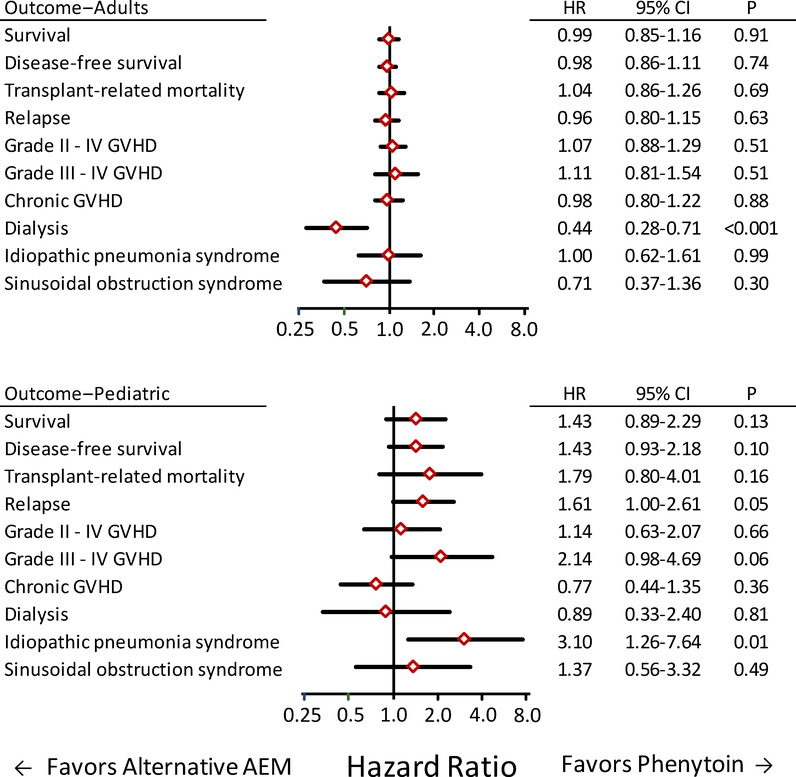
Figure 5. Most outcomes did not differ between patients who received alternative AEMs compared to those who received phenytoin in adult (top) and pediatric (bottom) patients after conditioning with IV BU followed by CY for treatment of AML, CML, MDS or lymphoid malignancy.
Diamonds indicate the hazards ratio point estimates for outcomes when results for the alternative AEM group were compared to those for the phenytoin group. Bars indicate the 95% confidence intervals. Each statistical model included risk factor covariate adjustments derived from the entire cohort. The number of pediatric patients who received oral BU is too small for an informative comparison with adult patients who received oral BU.