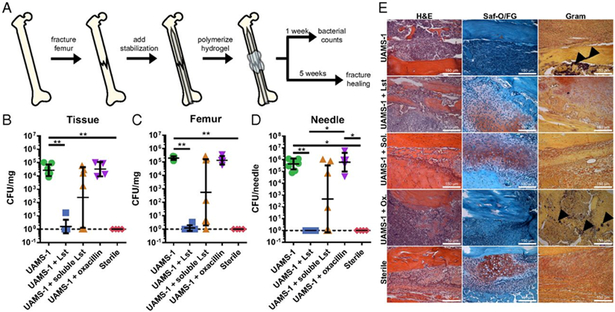
Figure 3.
(a) Schematic diagram detailing the model set-up for encapsulation of lysostaphin within a hydrogel matrix for localized infection mitigation and fracture healing in a bone defect model. (b) Bacterial counts 7 days postfracture of the tissue, femur, and stabilization needle for the following conditions: bacterial strain within hydrogel (UAMS-1), bacteria and lystostaphin within hydrogel (UAMS-1 + Lst), bacteria and lysostaphin without crosslinked hydrogel (UAMS-1 + soluble Lst), bacteria and prophylactic antibiotic injections (UAMS-1 + oxacillin), and a sterile control (sterile). (c) Histological sections of femurs stained with H&E, Saf-O/FG, and Gram. Arrows indicate sites of gram-positive bacteria. Reproduced with permission [71].