Figure 7. Hypothetical model for phosphoregulation of A. fumigatus CrzA during calcium stress.
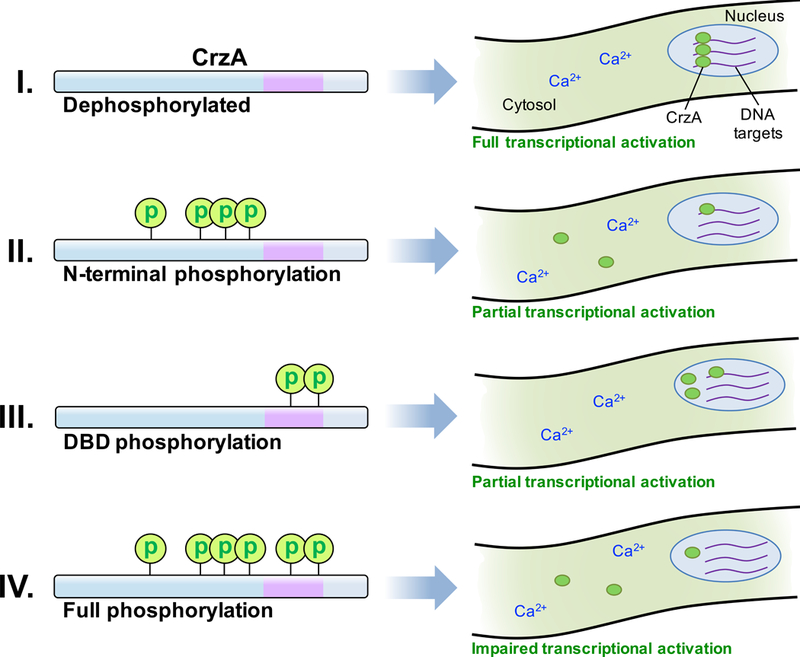
(I) When fully dephosphorylated in the presence of a high calcium ion concentration, CrzA is in the most active state, strongly localized to the nucleus and able to efficiently interact with the promoters of target genes to enable transcription following calcium stimulation. (II) When the N-terminal regulatory domain is phosphorylated, nuclear translocation is attenuated, but some protein may still enter the nucleus and bind efficiently to target genes. (III) When the DBD is phosphorylated, CrzA translocates to the nucleus, but efficiency of binding to target genes may be reduced. The high amount of nuclear protein present may enable sufficient expression of target genes. (IV) When CrzA is fully phosphorylated, both nuclear translocation and DNA-binding efficiency may be impaired, leading to insufficient activation of target gene expression and consequent phenotypic defects.
