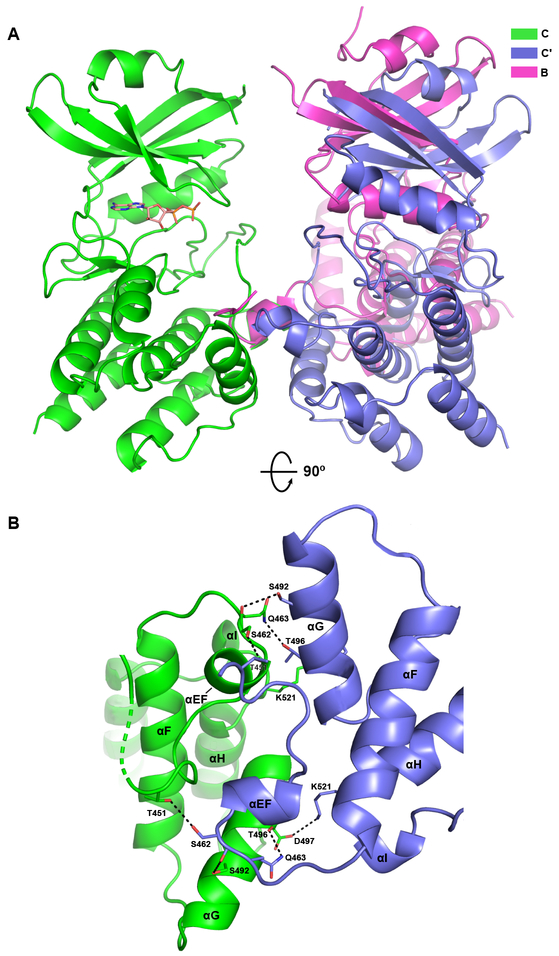
Figure 4. Structure of the PKR kinase face-to-face dimer without exchange.
Two symmetry-related C chains of the AMPPNP complex of PKR kinase forming a FTF dimer without exchange of activation segments are depicted using the color scheme from Figure 1. The chains are referred to as C and Cʹ. A) Comparison of the FTF interfaces. The A:B dimer with exchange and the C:Cʹ dimer without exchange were aligned on the A and C protomers on the left, treating the dimers as rigid units. Relative to the Cʹ protomer, the B protomer is rotated by 38°. The bound nucleotide in chain C is depicted in stick representation. B) Detailed view of the interactions stabilizing the interface. The orientation corresponds to a 90° rotation of the structure depicted in part A. Key side chain and main chain atoms are rendered as sticks. Hydrogen bond and salt-bridge interactions are denoted by dashed lines.