Abstract
The relatively low long-term survival rate of lung transplants as compared to other organ recipients serves as an impetus to identify potential lung dysfunction as early as possible. There is an association between donor heavy alcohol use and acute lung injury in the lung allograft after transplant, known as primary graft dysfunction. Excessive alcohol use (EAU) can induce pulmonary immune dysregulation in response to an infection. Antimicrobial peptides (AMPs) are an important component of the innate immune response to pulmonary infections, but the impact of EAU on AMPs in the allograft lung has not been evaluated. Our hypothesis is that specific lung AMPs, LL-37, α-defensin-1,2,3, and β-defensin-2, are dysregulated in the lungs from organ donors who had EAU. In this prospective observational investigation, we measured AMPs via ELISA and inflammatory cytokines via multiplex bead array, in bronchoalveolar lavage (BAL) fluid of lung allograft donors, comparing results based on their alcohol consumption. LL-37 levels in lung donors with EAU were found to be increased compared to nondrinker (ND) donors [median 7.7 ng/ml (IQR 4.1–37.0) vs 2.3 ng/ml (IQR 1.1–7.9), p=0.004] whereas α-defensins-1,2,3 was decreased only in the presence of an infection in donors with EAU compared to ND donors [median 2.2 ng/ml (IQR 1.6–2.4) vs 3.2 ng/ml (IQR 2.3–3.8), p=0.049]. There was no difference in β-defensin-2 levels. Gene expression levels of these AMPs were not different. Elevated levels of CXCL8 were noted in bronchial washings of donors with EAU compared to ND donors, [median 4372 pg/ml (IQR 3352–13180) vs 867.3 pg/ml (IQR 163.6–3675), p=0.04] suggesting a potentially heightened inflammatory response. At 1 month post-transplant, LL-37 and CXCL8 levels are decreased compared to levels at time of transplant. In lung donors with EAU, LL-37 and α-defensins-1,2,3 dysregulated levels in the presence of an infection may be a harbinger of dysfunction of the lungs through the transplant process.
Keywords: human antimicrobial peptides; LL-37; α-defensin-1,2,3; β-defensin-2; lung allografts; excessive alcohol use
Introduction
The pulmonary innate immune response is particularly important as the primary defense from the constant inhalational exposure of harmful pathogens and debris. Individuals with excessive alcohol use (EAU), either through intermittent binge drinking or chronic heavy daily use, have an increased risk of pulmonary infections (Jacobson, 1992; Jong, Hsiue, Chen, Chang, & Chen, 1995; Mehta & Guidot, n.d.; Moss & Burnham, 2006; Rehm et al., 2009; SAMOKHVALOV, IRVING, & REHM, 2010). One possible explanation for this observation is a dysfunctional pulmonary immune response to infections in people with EAU. Dramatic changes in the pulmonary innate immune response have been detected in those with EAU, including impairment of phagocytosis of bacteria by macrophages and neutrophils, reduced ciliary motion, decreases in the inflammatory response and the ability to recruit immune cells to clear infection from the airway, and reduced migration and adhesion of neutrophils (Curtis, Hlavin, Brubaker, Kovacs, & Radek, 2014; Gee, Kaskin, Duncombe, & Vassallo, 1974; Goral, Karavitis, & Kovacs, n.d.; Johnson, 1975; Kershaw & Guidot, 2008; Moss & Burnham, 2006; Reynolds, 1995; Rimland, n.d.; Sisson, 1995; Yeligar et al., 2016).
Lung transplantation is a complicated and aggressive therapy for advanced lung disease. Currently, the impact of excessive alcohol consumption on organ donors remains unclear. Furthermore, research investigating the effects of EAU on the pulmonary innate immune response in the context of lung transplantation is scarce, thus limiting our understanding of the effects of EAU on this crucial defense system. There is an increased risk of primary graft dysfunction (PGD) in lung transplant recipients who received lungs from donors with heavy alcohol use (Lowery et al., 2014; Pelaez et al., 2015). PGD is an acute lung injury occurring within the first 72 hours post lung transplant, and the exact mechanism as to why recipients whose donors drank alcohol excessively developed this injury is unclear, but may be in part related to the increased risk of pulmonary infections and perhaps an abnormal pulmonary immune response in donors with EAU.
Antimicrobial peptides (AMPs) are an important and long-standing component of the pulmonary innate immune response. AMPs are cationic peptides that have been shown to permeabilize the negatively charged bacterial membranes thus resulting in direct pathogen killing (Sawyer, Martin, & Hancock, 1988; Viljanen, Koski, & Vaara, 1988). There are three AMPs that are well known to be produced in the lung: LL-37(also called human cathelicidin, or hCAP18: human cationic antimicrobial peptide 18), α-defensin-1,2,3, and β-defensin-2 (Hiemstra, Amatngalim, Van Der Does, & Taube, 2016; Seiler, Bals, & Beisswenger, 2016). Additionally, the influence of AMPs is amplified by inducing indirect effects on pathogens by mediating inflammation such as the recruitment of neutrophils and monocytes, promoting adaptive immune responses, reducing inflammatory injury, promoting phagocytosis, and binding to DNA and RNA (Bals, 2000; Tecle, Tripathi, & Hartshorn, 2010). Anderson et al. identified a dysregulation of AMPs in lung transplant recipients diagnosed with bronchiolitis obliterans syndrome, otherwise known as chronic rejection, and others have noted alterations in AMPs in other lung diseases including chronic obstructive pulmonary disease, cystic fibrosis, and asthma (Anderson et al., n.d.; Hiemstra et al., 2016; Lecaille, Lalmanach, & Andrault, 2016; Stjärne Aspelund et al., 2017). These alterations in AMPs implicated in a several pulmonary diseases, in combination with the increased predisposition to pulmonary infections in EAU, of which AMP are a vital component in infection containment, led us to hypothesize that lung donors with EAU will have an altered pulmonary innate immune response, including alterations in pulmonary AMPs and inflammatory mediators.
MATERIALS and METHODS
Adult patients, ages ≥18 years old, who underwent lung transplantation from February 1, 2014 through May 2018 at Loyola University Medical Center, in Maywood, IL, were invited to participate in this study. Those who agreed to participate were included in this investigation. The investigation was approved by the Loyola University Chicago Health Science Division Institutional Review Board LU204490. Clinical and demographic information along with blood and bronchial washings were collected from lung allograft donors at time of procurement and brought back to Loyola University Chicago for processing and analysis.
Classification of Donor Alcohol Status
Donor health histories were acquired from the donor charts and made available to the recipient’s transplant center by the United Network of Organ Sharing (UNOS). Staff from the local organ procurement organization (OPO) administered the “Medical History& Behavioral Risk Assessment Questionnaire” to donor families prior to procurement of the donor organs and this questionnaire included information about the donor’s alcohol consumption history. The definition of EAU utilized in this investigation is 8 or more drinks per week for females and 15 or more drinks per week for males (“CDC - Fact Sheets-Alcohol Use And Health - Alcohol,” n.d.). In addition to alcohol consumption history, we utilized the direct alcohol biomarker phosphatidylethanol (PEth) as an objective measure for recent and excessive alcohol use. PEth is formed as a reaction between alcohol and the phospholipid membrane in the red blood cell (Cabarcos et al., 2013). Measurable in the blood for 2–3 weeks after heavy alcohol consumption, it has a 99% sensitivity and 100% specificity at detecting recent alcohol consumption (ARADOTTIR, ASANOVSKA, GJERSS, HANSSON, & ALLING, 2006). In classifying donors as EAU, we utilized donor alcohol history plus a PEth cutpoint >84ng/ml (Lowery, Yong, Cohen, Joyce, & Kovacs, 2018). We also obtained additional laboratory evidence of donor alcohol consumption through measures of liver biomarkers including aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), and percent carbohydrate deficient transferrin (%CDT). Clinical and demographic information collected from patient death. Nondrinkers (ND) were defined as having answered no charts included gender, age, smoking history, and cause of alcohol use on the Medical History & Behavioral Risk Assessment Questionnaire, in addition to a negative PEth. Social drinkers and/or moderate drinkers were not included in this investigation. Several of the donors had conflicting data in regards to their alcohol consumption, for example, a negative alcohol consumption history and a positive PEth. In those cases we were unable to accurately categorize and quantify the donor’s recent alcohol use as none or excessive and therefore the donor was excluded.
Bronchoalveolar lavage (BAL) fluid
Immediately prior to procurement of the lungs, bronchoalveolar lavage fluids were collected from the upper airways. The bronchoalveolar lavage fluid was then placed on ice and transported back to the laboratory where the BAL fluid was filtered with a 100 μM cell strainer (FisherScientific, Waltham, MA, USA) and flow-through was centrifuged at 1,200 rpm for 5 minutes at 4°C. BAL fluid supernatant was aliquoted into 250 μl volumes and stored at −80°C until time of analysis. Cell pellets were aliquoted as 1 × 106 cells in Trizol (FisherScientific, Waltham, MA, USA) and stored at −80°C until further processing. After transplant, recipients had BAL fluid collected 1–2 months after transplant, and samples were processed and stored in the same manner.
Respiratory Culture
Prior to procurement, the local OPO collects blood, urine, and BAL fluid for cultures in order to detect any active infections in the organ donor. Any pathologic bacterial growth, defined as known pathogens growing at >100,000 colony forming units (CFU) was considered positive in BAL fluid. In order to assess the pulmonary immune response to the presence of infection ND donors and donors with EAU were further categorized by whether or not their pre-procurement culture was positive for a pathologic bacteria.
Enzyme-linked immunosorbent assays (ELISA)
LL-37 (Hycult,Biotech, Netherlands), α-defensins-1,2,3 (Hycult Biotech, Netherlands), and β-defensin-2 (Pepro-Tech, Rocky Hill, NJ, USA) levels were analyzed as 50 μl of BAL fluid supernatant combined with 50 μl sample diluent from the above commercially available kits according to the manufactures’ instructions (Mallia et al., 2012). All measurements were performed twice for the same sample and results were averaged.
Human Cytokine
Cytokine concentrations in BAL fluid were measured in a 1:3 dilution by Bio-Rad Multiplex Assays (BioRad, Hercules, CA, USA) according to the manufacturer protocol (O’Halloran et al., 2016). All samples were assayed in duplicate and results were analyzed using the Bio-Plex manager software version 6.1. Eight cytokines were measured using a magnetic bead assay onto which detection antibodies are attached (BioRad Multiplex): interleukin 1 beta (IL-1β), interleukin 5 (IL-5), interleukin 6 (IL-6), interleukin 8 (CXCL8), interleukin (IL-12(p70)), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon gamma (IFN-γ), and monocyte chemoattractant protein 1 (CCL2).
RNA extraction and cDNA synthesis
Total RNA isolation was performed using 1 ml Trizol per 106 cells homogenized with 5 seconds’ sonication (GE Ultrsonic Processor 20 khz, Model GE50) and following manufacturer’s instructions (Rio, Ares, Hannon, & Nilsen, 2010). Samples were resuspended in 20 μl sterile MilliQ water and run on an automated gel (Agilent Technologies, Wood Dale, IL, USA) following manufacturer’s instructions and selected samples that reached a level 5 RNA Integrity Number (RIN) for further processing. cDNA was synthesized from 100 ng total RNA by using iScript cDNA synthesis kit (BioRad, Hercules, CA, USA).
RT-PCR
Quantitative RT-PCR was performed by using 4 μl diluted cDNA and 16 μl of Taqman Fast universal PCR master mix (Thermo Fisher Scientific, Waltham, MA, USA) containing specific primers (20μM) and probes (5μM) for GAPDH as endogenous control and LL-37, α-defensins-1,2,3, and β-defensin-2 (Hs99999905m1, Hs00189038m1, Hs00234383m1, Hs00823638m1). CD86, a macrophage marker, was also measured (Hs01567026m1). PCR reactions were carried out on an Applied Biosytems Step One Real Time PCR system (Thermo Fisher Scientific, Waltham, MA, USA). The ΔΔCT method was used to quantify the mRNA expression levels of genes which were normalized to the housekeeping gene GAPDH (HRPT1, 18s, TBP, GAPDH tested) in non alcohol user samples. Only RNA that showed intact RNA as determined by BioAnalyzer gel electrophoresis were used for RT-PCR quantification of gene expression levels.
Statistical Analysis
Standard descriptive statistics, including medians and interquartile ranges (IQR) for continuous variables or frequencies and percent’s for categorical variables, were used to describe clinical and demographic characteristics of the study population. Bivariate comparisons among donor alcohol groups were performed using Pearson’s χ2 for categorical variables and Kruskal-Wallis tests for nonparametric continuous variables. Analysis of two treatment groups, ND vs EAU, and two time points, transplant vs 1 month post-transplant, was performed using two-way analysis of variance (ANOVA) with Bonferroni’s multiple comparisons test. In comparing levels of AMPs at transplant to those 1 month post-transplant, a stratified approach utilizing nonparametric tests was utilized. Nonparametric tests were used to analyze protein and relative RNA levels. The threshold for significant p-values is <0.05. Data were analyzed and processed using GraphPad Prism 4.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
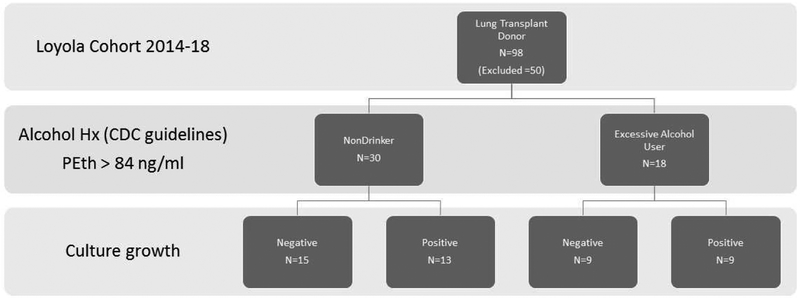
There were 98 lung donors from 2014 thru 2018, of which 48 were included in the study cohort. There were 30 nondrinkers (ND) and 18 EAU (Figure 1). 50 moderate drinkers or donors with inconsistent and/or missing alcohol histories and alcohol biomarkers, were excluded from the study. Lung allograft donor demographic and clinical information is contained in Table 1. Between the two groups, ND and EAU, there were no differences in sex, age, cause of death, presence of lung infection, nor smoking (Table 1). GGT and %CDT were elevated in those donors with EAU. Table 2 contains the pathogens that were positive in pre-procurement cultures. Missing from the UNOS database are culture results for 2 individuals within the ND cohort.
Figure 1.
Stratification of study participants. 98 lung transplant donors at study site with 48 donors included. Two ND samples had missing pathologic culture information. Hx=history. PEth=phosphatidylethanol.
Table 1.
Cohort Demographics: Nondrinker versus Excessive Alcohol User
| Nondrinker N=30 |
Excessive Drinker (EAU) N=18 |
p-value | |
|---|---|---|---|
| Donor Age, median (IQR) | 25.5 (20–39) | 39.5 (23–47) | 0.16 |
| Donor Gender Male, % (No.) | 53 (16) | 72 (13) | 0.19 |
| Donor Smoking, % (No.) | 41 (12) | 61 (11) | 0.19 |
| UNOS Infection, % | 43 (13) | 50 (9) | 0.80 |
| GGT, median (IQR) | 20.5 (12–39) | 48 (26–373) | 0.009 |
| ALT, median (IQR) | 31 (18–94) | 36 (23–122) | 0.33 |
| AST, median (IQR) | 42 (24–109) | 64 (20–315) | 0.23 |
| AST/ALT, median (IQR) | 1.07 (0.83–2.14) | 1.55 (0.98–2.0) | 0.41 |
| %CDT, median (IQR) | 1.45 (1.0–2.15) | 1.8 (1.6–2.7) | 0.02 |
| PEth, median (IQR) | 0 | 95 (62–258) | <0.001 |
| Cause of Death, % (No.) | 0.21 | ||
| Gun Shot Wound | 23 (7) | 22 (4) | |
| ICH | 40 (12) | 22 (4) | |
| Blunt Trauma | 13 (4) | 39 (7) | |
| Anoxia | 23 (7) | 17 (3) |
All nonparametric variables assessed with Mann-Whitney or Pearson’s chi-square
GGT: gamma-glutamyl transferase, AST: aspartate aminotransferase, ALT: alanine aminotransferase, CDT: carbohydrate deficient transferrin, ICH: intracerebral hemorrhage, IQR: interquartile range
Table 2.
Donor preoperative positive culture
| Positive Culture | N |
|---|---|
| Acinetobacter baumannii | l |
| Aspergillus species | l |
| Citrobacter koseri | l |
| Enterobacteriaceae | 3 |
| Haemophilus influenza | 6 |
| Klebsiella oxytoca | 3 |
| Klebsiella pneumonia | 1 |
| Rothia mucinaginosa | 2 |
| Serratia marcescens | 1 |
| Staphylococcus aureus | 23 |
| Staphylococcus epidermis | 2 |
| Streptococcus pneumonia | 1 |
Pathologic bacteria growth detected in BAL fluid obtained 1–2 days prior to procurement of donor lungs.
AMP protein and gene expression
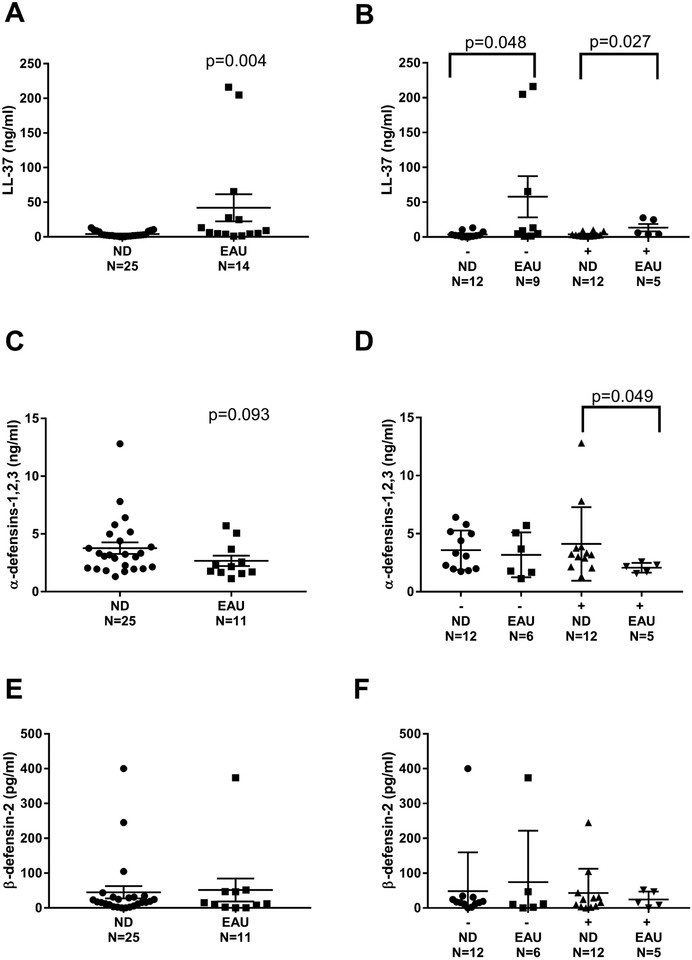
AMP measurements are displayed in Figure 2. LL-37 was elevated in donors with EAU as compared to ND donors [median 7.7 ng/ml (IQR 4.1–37.0) vs 2.3 ng/ml (IQR 1.1–7.9), p=0.004] (Figure 2A). In order to assess for pulmonary innate immune system dysregulation, we next looked at AMPs in the presence or absence of infection based on pre-procurement cultured BAL fluid. Those individuals with EAU had higher (3-fold and 4-fold respectively) BAL levels of LL-37 regardless of presence or absence of infection (Figure 2B). Comparatively, levels of α-defensins-1,2,3 only differed in donors with EAU and a positive culture for pathologic bacteria compared to lung donors who were ND with a positive culture, [median 2.2 ng/ml (IQR 1.6–2.4) vs 3.2 ng/ml (IQR 2.3–3.8), p=0.049] (Figure 2D). There was no difference in the levels of β-defensin-2, either in total or in the presence or absence of infection (Figure 2E, 2F).
Figure 2.
Levels of AMPs in BAL fluid at transplant. A) LL-37 B) LL-37 with negative or positive culture growth C) α-defensin-1,2,3 D) α-defensin-1,2,3 with negative or positive culture growth E) β-defensin-2 F) β-defensin-2 with negative or positive culture growth. Mann-Whitney test was performed between groups; p<0.05 was considered significant. Tx=transplant. ND=nondrinker. EAU=excessive alcohol user. (−)=negative culture. (+)=positive culture.
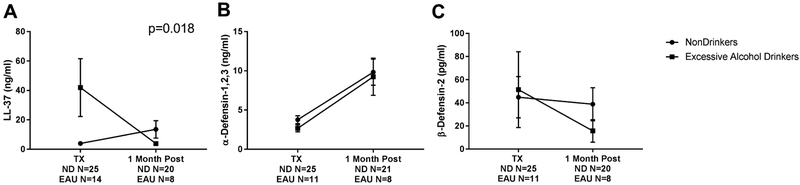
One month post lung transplant, AMPs were measured in the BAL fluid from recipients. LL-37 was the only AMP that revealed a significant time point × alcohol exposure interaction, F(1,63)=5.955, p=0.018, as measured by a two-way ANOVA (Figure 3A). Next, we stratified the analysis comparing changes in each group, ND and EAU, from transplant to 1 month post-transplant, and stratified again to look for differences at 1 month transplant between those with ND and EAU. Levels of LL-37 in ND at 1 month post-transplant compared to levels at transplant slightly increased [median 2.336 ng/ml (IQR 1.148–7.877) vs 5.953 ng/ml (IQR 2.397–12.6), p=0.025] (Figure 3A). Whereas levels of LL-37 in EAU at 1 month post-transplant compared to levels at transplant significantly decreased [median 7.664 ng/ml (IQR 4.109–37.04) vs 3.735 ng/ml (IQR 0.6595–6.557), p=0.035] (Figure 3A). At 1 month post-transplant, BAL levels of LL-37 in EAU were not different than levels at 1 month in ND [median 5.953 ng/ml (IQR 2.397–12.6) vs 3.735 ng/ml (IQR 0.6595–6.557), p=0.1816] (Figure 3A). The second AMP measured at one 1 post lung transplant was α-defensins-1,2,3. For ND, BAL levels of α-defensins-1,2,3 at 1 month post-transplant compared to levels at transplant more than doubled [median 3.182 ng/ml (IQR 2.013–4.692) vs 8.216 ng/ml (IQR 3.015–15.05), p=0.0043] (Figure 3B). Likewise levels of α-defensins-1,2,3 in BAL of EAU patients at 1 month post-transplant were elevated by more than a 4-fold increase [median 2.169 ng/ml (IQR 1.673–3.683) vs 9.29 ng/ml (IQR 2.713–15.89), p=0.020] (Figure 3B). At 1 month post-transplant, BAL levels of α-defensins-1,2,3 in EAU were similar to levels at 1 month in ND [median 8.216 ng/ml (IQR 3.015–15.05) vs 9.29 ng/ml (IQR 2.713–15.89), p=0.0.943] (Figure 3B). We also measured BAL levels of β-defensin-2. In ND, at 1 month post-transplant BAL β-defensin-2 levels were similar to the concentrations seen at time of transplant [median 17.39 pg/ml (IQR 7.558–31.03) vs 4.186 pg/ml (IQR 4.186–67.94), p=0.316] (Figure 3C). Likewise, levels of β-defensin-2 in EAU at 1 month post-transplant did not differ from concentrations at time of transplant [median 11.88 pg/ml (IQR 2.367–46.91) vs 4.186 pg/ml (IQR 4.186–11.73), p=0.474] (Figure 3C). At 1 month post-transplant, levels of β-defensin-2 in EAU were similar to levels at 1 month in ND [median 4.186 pg/ml (IQR 4.186–67.94) vs 4.186 pg/ml (IQR 4.186–11.73), p=0.7459] (Figure 3C). Concisely stated, the pulmonary inflammatory milieu changed at 1 month post-transplant in terms of levels of LL-37 and α-defensins-1,2,3, but not β-defensin-2.
Figure 3.
Levels of AMPs in BAL fluid at transplant and 1 month post-transplant. A) LL-37 B) α-defensin-1,2,3 C) β-defensin-2. Significant differences are listed, two way ANOVA with p<0.05 considered significant. ND=nondrinker. EAU=excessive alcohol user. (−)=negative culture. (+)=positive culture.
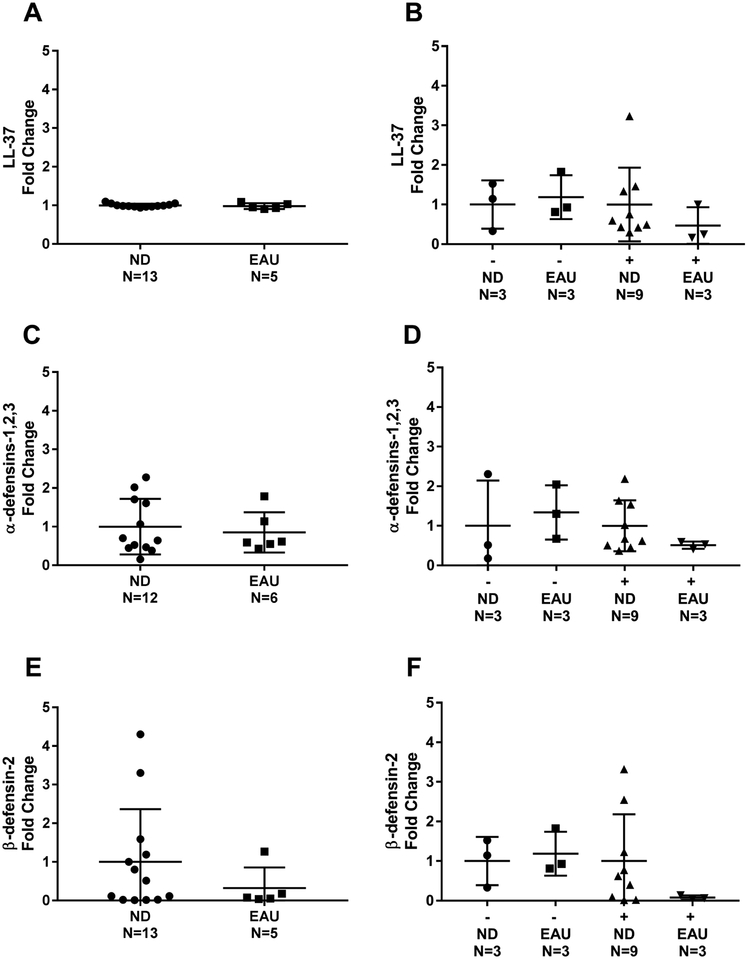
There was no difference in the gene expression levels of AMPs at time of transplant in ND compared to EAU (Figure 4). Amongst donors with EAU and positive culture, the gene expression of all three AMPs trended lower than the other three groups. There were similar rates of intact RNA with a RNA Integrity Number (RIN) >5: 48% in nondrinkers and 43% in EAU.
Figure 4.
ND and EAU Lung Donor BAL Cell Lysate AMP Gene Expression at transplant. Relative fold change in the expression of gene of interest is shown A) LL-37 B) LL-37 with negative or positive culture growth C) α-defensin-1,2,3 D) α-defensin-1,2,3 with negative or positive culture growth E) β-defensin-2 F) β-defensin-2 with negative or positive culture growth. Differences were not significant; Mann-Whitney test was performed between groups. ND=nondrinker. EAU=excessive alcohol user. (−)=negative culture. (+)=positive culture.
Lung Cytokines and Macrophage Marker CD86
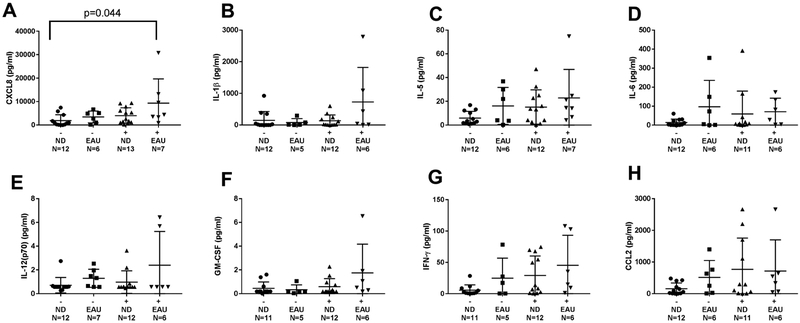
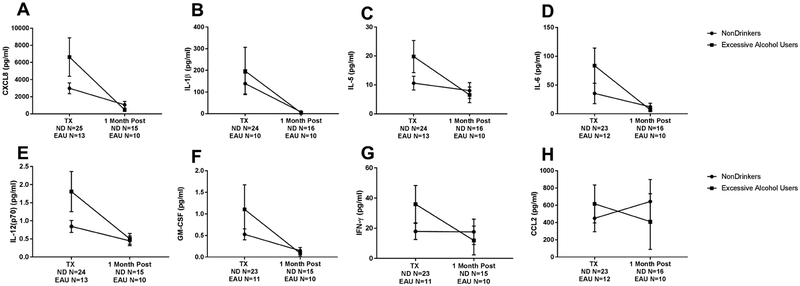
Of the eight cytokines analyzed, there was 5-fold higher levels of CXCL8 in BAL from EAU donors compared to the ND donors, [median 4372 pg/ml (IQR 3352–13180) vs 867.3 pg/ml (IQR 163.6–3675), p=0.044] (Figure 5). There was a nonsignificant increase in levels of inflammatory cytokines (IL-1β, IL-5, IL-12(p70), GM-CSF), and IFN-γ) in EAU donors in the presence of an infection at time of lung transplant. Whereas at the 1 month post-transplant time point,CXCL8, IL-1β, IL-5, IL-6, IL-12(p70), GM-CSF, and IFN-γ had significantly decreased from the time of transplant to levels similar to ND donors (Figure 6). In contrast, BAL levels of CCL2 decreased in levels at 1 month compared to transplant levels [median 439.9 pg/ml (IQR 53.1–731.8) vs 109.8 pg/ml (IQR 24.92–155.1), p=0.160], whereas ND donors CCL2 levels stayed the same from transplant to 1 month post-transplant (Figure 6H). At time of transplant, expression of CD86 was similar in EUA and nondrinkers [median 0.5459 (IQR 0.3643–1.972) vs 0.478 (IQR 0.2905–2), p=0.682], signifying equal amounts of macrophages in each group.
Figure 5.
Levels of cytokines in BAL fluid at transplant with negative or positive culture growth. Cytokine CXCL8 showed increase in terms of positive culture and EAU (A). Cytokines showing no significant change B) IL-1β C) IL-5 D) IL-6 E) IL12(p70) F) GM-CSF G) IFN-γ H) CCL2. Kruskal-Wallis test with multiple comparisons was performed. Data are presented as median and interquartile range. ND=nondrinker. EAU=excessive alcohol user. (−) =negative culture. (+)=positive culture.
Figure 6.
Levels of cytokines in BAL fluid at transplant and at 1 month post-transplant. Cytokines showed significant decrease (A) CXCL8, p<0.0001 B) IL-1β, p=0.040 C) IL-5, p<0.0001 D) IL-6, p<0.0001 E) IL12(p70) p<0.0001 F) GM-CSF, p<0.0001 and G) IFN-γ, p<0.00001. H) CCL2 was increased for EAU and decreased for nondrinkers at one month post-transplant p=0.002. 2 way ANOVA was performed with p<0.05 considered significant. Tx=transplant. ND=nondrinker. EAU=excessive alcohol user. (−)=negative culture. (+)=positive culture.
DISCUSSION
There is increasing evidence demonstrating that the pulmonary immune response is altered in lungs of excessive alcohol users (Goral et al., n.d.; Moss & Burnham, 2006). Our study contributes to this body of knowledge by observing that AMPs, LL-37 and α-defensin-1,2,3, components of the innate immune response, are altered in lungs from donors with EAU as early as time of transplant. We also detected that the alterations in AMP levels are not due to a difference in gene expression in airway cells between EAU and ND. In addition, we showed that levels of CXCL8, an innate immune cleaved and then secreted inflammatory pro-protein, is elevated in EAU lung donors with a positive culture. One month post-transplant, it appears that both levels of AMPs and CXCL8 return to levels comparable in the ND lung allograft donor.
To our knowledge, this is the first human study to assess the impact of excessive alcohol exposure on lung allograft expression of AMPs. Additionally, it is the first to compare lung allograft AMPs expression in patients with different alcohol exposures longitudinally, at transplant and 1 month following lung transplant. Importantly, there were significant changes in these AMPs in response to excessive alcohol use. Ostaff and colleagues proposed that alcohol induced oxidative stress leads to an AMP dysregulation, which is protective, and may restore the weakened epithelial defense during alcohol-mediated damage (Ostaff et al., 2015). On the other hand, this same group also suggested that a change in AMPs may represent the first symptom of a detrimental epithelial transformation in individuals with heavy chronic alcohol use (Ostaff et al., 2015). In either case, our work corroborates research preformed in other organ systems, namely the gastrointestinal track and the liver, identifying changes in AMPs associated with excessive alcohol exposure (Ibusuki et al., 2017; Ostaff et al., 2015). One exception is a study that saw no differences in LL-37 levels in the cell pellets of BAL fluid from people with alcohol use disorders (AUD) (Ogunsakin, Hottor, Mehta, Lichtveld, & McCaskill, 2016)
As described herein, 1 month following lung transplant, LL-37 levels in the BAL fluid of those with EAU donors decreased to similar levels as those seen in recipients with ND donors. Distinctly, α-defensins-1,2,3 changed 1 month post-transplant in the same trajectory in both recipients who had EAU donors and ND donors. The majority of cells in the BAL fluid are known to be macrophages (Byrne, Mathie, Gregory, & Lloyd, n.d.; Tiroke, Bewig, & Haverich, 1999). One of the primary sources of innate immune mediators in the lungs are macrophages, and we noted similar expression of the macrophage marker CD86 in both EAU and ND. The lack of change in cells expressing CD86 may signify that the number or distribution of macrophages did not differ in between our samples. This may implicate other cellular sources of AMPS besides macrophages such as neutrophils.
Furthermore, the cells source of AMPs may be changing from lung donor to lung recipient. The decline in LL-37 levels at 1 month post-transplant in recipients whose donors had EAU is intriguing because donors cells remain present in the lungs, and it is only at 3 months post lung transplant that lung recipient cells replace the majority of cells obtained in BAL fluid (Paradis et al., n.d.; Tiroke et al., 1999). Our data suggests changes in AMPs prior to this 3 month time period, which may implicate other sources of AMPs besides donor airway immune cells, such as epithelium. An additional important consideration is that recipients are instructed to remain abstinent from alcohol following lung transplant, and therefore alcohol exposure during this time period is zero. Thus, these variations in BAL fluid AMP levels suggest evidence of selective and differential innate immune response to excessive alcohol consumption prior to transplantation and henceforth to changing pulmonary immune environment at 1 month post-transplant.
These findings are compelling in light of previously published reports that there is an increase in LL-37 and α-defensins-1,2,3, as well as no change in β-defensin-2 in patients presenting with bronchiolitis obliterans syndrome (BOS), or chronic rejection of lung allografts (Anderson et al., n.d.). A long standing concern in the pulmonary transplant field is the mechanism causing the development of BOS in the long term. It is not known why AMPs are increased in cases of chronic rejection of lung allografts. One plausible mechanism for lung dysfunction may lie in the earlier donor exposure to excessive alcohol consumption. Our study provides evidence that donor exposure to excessive alcohol is associated with altered levels of LL-37 and α-defensins-1,2,3. It is unknown if donor EAU contributes to the development of BOS, although it is a known risk factor for developing PGD, which can raise the risk of developing BOS long-term (Lee, Christie, & Keshavjee, 2010; Lowery et al., 2014).
Our research found no difference in gene expression in any of the three AMPs of interest, which points to other mechanisms besides increased transcription, to explain increases in LL-37. Increases in measured levels of LL-37 may be due to an increase in processing of the pro-protein LL-37 and does perhaps does not require a change in mRNA (Park et al., 2016). LL-37 pro-protein is stored in various lung cells including alveolar macrophages, neutrophils, mast cells, and airway epithelial cells (Wah Anne Wellek Marion Frankenberger Pia Unterberger Ulrich Welsch Robert Bals, 2006). Upon immunological activation pro-protein LL-37 is cleaved into active LL-37 form and released into the alveolar space (Dürr, Sudheendra, & Ramamoorthy, 2006). Whereas a decrease in α-defensins-1,2,3 may result from a deficient release of the AMP from pulmonary neutrophil cells. Considering AMPs are released from cellular storage, BAL fluid supernatant is particularly useful in measuring extracellular active AMPs. Further research is required to determine the extent of the correlative relationship between mRNA and protein levels of AMPs from specific lung cells.
In this study, we also explored the effect of alcohol exposure on airway cytokines levels and identified an increase in CXCL8 in EAU lung donors. Individuals with EAU have increases in plasma levels of CXCL8 (Leclercq, De Saeger, Delzenne, de Timary, & Stärkel, 2014; Li et al., 2017). Unfortunately, these studies did not include lung measurements. Our work suggests that excessive alcohol exposure may serve as a deregulator of CXCL8 directly in BAL fluid. Comparably, O’Halloran et al. identified a 1.3 fold increase in airway CXCL8 contained within airway immune cells in people with AUD (O’Halloran et al., 2016). Moreover, human studies have shown a correlation between CXCL8 measured explicitly in BAL fluid with a diagnosis of acute lung injury syndrome (Allen & Kurdowska, 2014; Morrison, Pither, & Fisher, 2017). In addition, CXCL8 has been identified by the Lung Transplant Outcomes Group as a biomarker of PGD (FISHER et al., 2001; Morrison et al., 2017). Our current study suggests that exposure to excessive alcohol may instigate CXCL8-mediated immune dysregulation without the presence of acute lung injury nor PGD. Perhaps CXCL8 is a harbinger of potential lung injury. Furthermore, altered levels of CXCL8 may be connected to elevated airway levels of LL-37. Tjabringa et al. noted that in vitro addition of LL-37 to primary human bronchial epithelial cells from lung cancer patients as well as human mucoepidermoid lung carcinoma cell line NCI-H292, increased the levels of production of CXCL8 in a concentration and time dependent manner (Tjabringa et al., 2003). One unanswered question is whether elevated levels of LL-37 and CXCL8 prior to transplant could predispose recipients to the development of BOS or acute lung injury.
The mechanism responsible for the upregulation of immune mediators in EAU is not well understood. O’Halloran et al. proposed that an upregulation of innate mediators such as CXCL8 may be associated with a paradoxical downregulation of downstream receptors or signaling pathways to sufficiently complete the immune task (O’Halloran et al., 2016). Another possibility is that these innate immune mediators are increased because they are not able to achieve their function as has been proposed in the case of increased LL-37 and cystic fibrosis cases (Chen, Schaller-Bals, Paul, Wahn, & Bals, 2004). Our data support this assertion, as altered levels were primarily seen in the presence of infection. Another scenario proposes that an aberrant signaling overstimulation induces these increased levels of LL-37, which may then prove to be cytotoxic in lung transplant as shown in other lung diseases such as asthma (Ashitani, Matsumoto, & Nakazato, 2005; Chen et al., 2004; Hiemstra, 2015; Lecaille et al., 2016; Liu, Xiao, Brown, Ritter, & Schroeder, 2012; Schögler et al., 2016). To elucidate the reason for dysregulation, as well as putative downstream effects, we are in the process of following long-term outcome data to determine if the alterations seen in the donor have a long term-term impact on outcomes.
The major limitation of our investigation is a small sample size. Despite collection of 98 lung donors into this cohort, only 48 met inclusion criteria. In particular, we noted trends developing in gene expression and cytokine levels that may be limited by the small available sample size in this cohort. Of the 41 samples from which RNA was extracted, only 19 had intact RNA necessary for gene expression analysis. Furthermore, the coordination and prompt obtainment of study sample will remain a persistent challenge given the logistics of time and travel in organ procurement. Specifically, measurement of gene expression on donor samples is highly dependent upon the logistics of sample acquisition and timing of sample processing. Oftentimes, samples are bought back from the donor hospital several hours after obtaining the sample due to the distance of the donor from the recipient hospital. Despite this limitation, we felt it was important to report the findings that we observed, as there are not previous transplant investigations noting the impact of alcohol on airway AMPs.
In conclusion, we observed increased levels of the AMP LL-37 and CXCL8 in lung allograft donors with EAU. Decreased levels of α-defensins-1,2,3 were observed in donors with EAU in the presence of infection. Importantly we noted these alterations only in measured levels of secreted LL-37 and α-defensins-1,2,3 in BAL fluid whereas gene expression was not noted to be different. At 1 month post-transplant there were significant decreases in LL-37 and in CXCL8. The specific mechanism that triggers the immune system to increase levels of LL-37 or decreased α-defensins-1,2,3 at time of transplant has yet to be determined. It is possible that these identified innate immune dysregulations lead to a more pronounced lung allograft injury. Currently, there remains an imperative to improve lung transplant outcomes. Thus early identification of altered inflammation and AMPs would allow for potential remediation of any deficits in lung allograft function.
Highlights.
There are increased levels of LL-37 in lungs from donors with excessive alcohol consumption.
There are decreased levels of α-defensins-1,2,3 in lungs from donors with excessive alcohol consumption in the presence of infection.
Innate immune dysregulation occurs in excessive alcohol user donor lungs as evidenced by altered levels of antimicrobial peptides and CXCL8.
Not all lung antimicrobial peptides are dysregulated by excessive alcohol exposure in donors.
Altered antimicrobial peptides and cytokine levels normalized by one month following transplant.
Acknowledgements:
We would like to thank the patients and staff at Loyola University Medical Center and the lung transplant team their valuable help.
Funding Information and Conflict of Interest
This research was supported by K23AA022126 (EML), R01GM115257 (EJK), T32AA013527 (Choudhry, MA), T35 HL120835 (Zeleznik-Le, NJ)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest to disclose amongst the authors.
References
- Allen TC, & Kurdowska A (2014). Interleukin 8 and Acute Lung Injury. Archives of Pathology & Laboratory Medicine, 138(2), 266–269. 10.5858/arpa.2013-0182-RA [DOI] [PubMed] [Google Scholar]
- Anderson RL, Hiemstra PS, Ward C, Forrest IA, Murphy D, Proud D, … Fisher AJ (n.d.). Antimicrobial peptides in lung transplant recipients with bronchiolitis obliterans syndrome. 10.1183/09031936.00110807 [DOI] [PubMed] [Google Scholar]
- Aradottir S, Asanovska G, Gjerss S, Hansson P, & Alling C (2006). PHOSPHATIDYLETHANOL (PEth) CONCENTRATIONS IN BLOOD ARE CORRELATED TO REPORTED ALCOHOL INTAKE IN ALCOHOL-DEPENDENT PATIENTS. Alcohol and Alcoholism, 41(4), 431–437. 10.1093/alcalc/agl027 [DOI] [PubMed] [Google Scholar]
- Ashitani J, Matsumoto N, & Nakazato M (2005). Elevated Levels of Antimicrobial Peptides in Bronchoalveolar Lavage Fluid in Patients with Chronic Eosinophilic Pneumonia. Respiration, 74(1), 69–75. 10.1159/000090199 [DOI] [PubMed] [Google Scholar]
- Bals R (2000). Epithelial antimicrobial peptides in host defense against infection. Respiratory Research, 1(3), 5 10.1186/rr25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne AJ, Mathie SA, Gregory LG, & Lloyd CM (n.d.). Pulmonary macrophages: key players in the innate defence of the airways. 10.1136/thoraxjnl-2015-207020 [DOI] [PubMed] [Google Scholar]
- Cabarcos P, Cocho JÁ, Moreda A, Míguez M, Tabernero MJ, Fernández P, & Bermejo AM (2013). Application of dispersive liquid–liquid microextraction for the determination of phosphatidylethanol in blood by liquid chromatography tandem mass spectrometry. Talanta, 111, 189–195. 10.1016/j.talanta.2013.03.008 [DOI] [PubMed] [Google Scholar]
- CDC - Fact Sheets-Alcohol Use And Health - Alcohol. (n.d.). Retrieved June 1, 2018, from https://www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm [Google Scholar]
- Chen CI-U, Schaller-Bals S, Paul KP, Wahn U, & Bals R (2004). b-defensins and LL-37 in bronchoalveolar lavage fluid of patients with cystic fibrosis. Journal of Cystic Fibrosis, 3, 45–50. 10.1016/j.jcf.2003.12.008 [DOI] [PubMed] [Google Scholar]
- Curtis BJ, Hlavin S, Brubaker AL, Kovacs EJ, & Radek KA (2014). Episodic Binge Ethanol Exposure Impairs Murine Macrophage Infiltration and Delays Wound Closure by Promoting Defects in Early Innate Immune Responses. Alcoholism: Clinical and Experimental Research, 38(5), 1347–1355. 10.1111/acer.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürr UHN, Sudheendra US, & Ramamoorthy A (2006). LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochimica et Biophysica Acta, 1758(9), 1408–1425. 10.1016/j.bbamem.2006.03.030 [DOI] [PubMed] [Google Scholar]
- Fisher AJ, Donnelly SC, Hirani N, Haslett C, Strieter RM, DARK JH, & CORRIS PA (2001). Elevated Levels of Interleukin-8 in Donor Lungs Is Associated with Early Graft Failure after Lung Transplantation. American Journal of Respiratory and Critical Care Medicine, 163(1), 259–265. 10.1164/ajrccm.163.1.2005093 [DOI] [PubMed] [Google Scholar]
- Gee JB, Kaskin J, Duncombe MP, & Vassallo CL (1974). The effects of ethanol on some metabolic features of phagocytosis in the alveolar macrophage. Journal of the Reticuloendothelial Society, 15(1), 61–68. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4810602 [PubMed] [Google Scholar]
- Goral J, Karavitis J, & Kovacs EJ (n.d.). Exposure -dependent effects of ethanol on the innate immune system. Retrieved from https://www-ncbi-nlm-nihgov.flagship.luc.edu/pmc/articles/PMC2453223/pdf/nihms55629.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemstra PS (2015). Parallel activities and interactions between antimicrobial peptides and complement in host defense at the airway epithelial surface. Molecular Immunology, 68(1), 28–30. 10.1016/j.molimm.2015.07.030 [DOI] [PubMed] [Google Scholar]
- Hiemstra PS, Amatngalim GD, Van Der Does AM, & Taube C (2016). Antimicrobial Peptides and Innate Lung Defenses Role in Infectious and Noninfectious Lung Diseases and Therapeutic Applications. CHEST, 149(2), 545–551. 10.1378/chest.15-1353 [DOI] [PubMed] [Google Scholar]
- Ibusuki R, Uto H, Oda K, Ohshige A, Tabu K, Mawatari S, … Ido A (2017). Human neutrophil peptide-1 promotes alcohol-induced hepatic fibrosis and hepatocyte apoptosis. PLOS ONE, 12(4), e0174913 10.1371/journal.pone.0174913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JM (1992). Alcoholism and tuberculosis. Alcohol Health & Research World, 16(1), 39 Retrieved from http://flagship.luc.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=a9h&AN=9302010962&site=ehost-live [Google Scholar]
- Johnson WD (1975). Impaired defense mechanisms associated with acute alcoholism. Annals of the New York Academy of Sciences, 252, 343–347. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1096714 [DOI] [PubMed] [Google Scholar]
- Jong GM, Hsiue TR, Chen CR, Chang HY, & Chen CW (1995). Rapidly fatal outcome of bacteremic Klebsiella pneumoniae pneumonia in alcoholics. Chest, 107(1), 214–217. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7813281 [DOI] [PubMed] [Google Scholar]
- Kershaw CD, & Guidot DM (2008). Alcoholic lung disease. Alcohol Research & Health : The Journal of the National Institute on Alcohol Abuse and Alcoholism, 31(1), 66–75. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23584753 [PMC free article] [PubMed] [Google Scholar]
- Lecaille F, Lalmanach G, & Andrault P-M (2016). Antimicrobial proteins and peptides in human lung diseases: A friend and foe partnership with host proteases. 10.1016/j.biochi.2015.08.014 [DOI] [PubMed] [Google Scholar]
- Leclercq S, De Saeger C, Delzenne N, de Timary P, & Stärkel P (2014). Role of Inflammatory Pathways, Blood Mononuclear Cells, and Gut-Derived Bacterial Products in Alcohol Dependence. Biological Psychiatry, 76(9), 725–733. 10.1016/j.biopsych.2014.02.003 [DOI] [PubMed] [Google Scholar]
- Lee J, Christie J, & Keshavjee S (2010). Primary Graft Dysfunction: Definition, Risk Factors, Short- and Long-Term Outcomes. Seminars in Respiratory and Critical Care Medicine, 31(02), 161–171. 10.1055/s-0030-1249111 [DOI] [PubMed] [Google Scholar]
- Li W, Amet T, Xing Y, Yang D, Liangpunsakul S, Puri P, … Yu Q (2017). Alcohol abstinence ameliorates the dysregulated immune profiles in patients with alcoholic hepatitis: A prospective observational study. Hepatology, 66(2), 575–590. 10.1002/hep.29242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MC, Xiao H-Q, Brown AJ, Ritter CS, & Schroeder J (2012). Association of vitamin D and antimicrobial peptide production during late-phase allergic responses in the lung. Clinical & Experimental Allergy, 42(3), 383–391. 10.1111/j.1365-2222.2011.03879.x [DOI] [PubMed] [Google Scholar]
- Lowery EM, Kuhlmann EA, Mahoney EL, Dilling DF, Kliethermes SA, & Kovacs EJ (2014). Heavy Alcohol Use in Lung Donors Increases the Risk for Primary Graft Dysfunction. Alcoholism: Clinical and Experimental Research, 38(11), 2853–2861. 10.1111/acer.12553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EM, Yong M, Cohen A, Joyce C, & Kovacs EJ (2018). Recent alcohol use prolongs hospital length of stay following lung transplant. Clinical Transplantation, e13250 10.1111/ctr.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallia P, Footitt J, Sotero R, Jepson A, Contoli M, Trujillo-Torralbo M-B, … Johnston SL (2012). Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 186(11), 1117–1124. 10.1164/rccm.201205-0806OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AJ, & Guidot DM (n.d.). Alcohol Research: Current Reviews; Retrieved from https://www-ncbi-nlmnih-gov.flagship.luc.edu/pmc/articles/PMC5513688/pdf/arcr-38-2-243.pdf [PMC free article] [PubMed] [Google Scholar]
- Morrison MI, Pither TL, & Fisher AJ (2017). Pathophysiology and classification of primary graft dysfunction after lung transplantation. Journal of Thoracic Disease, 9(10), 4084–4097. 10.21037/jtd.2017.09.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss M, & Burnham EL (2006). Alcohol abuse in the critically ill patient. The Lancet, 368(9554), 2231–2242. 10.1016/S0140-6736(06)69490-7 [DOI] [PubMed] [Google Scholar]
- O’Halloran EB, Curtis BJ, Afshar M, Chen MM, Kovacs EJ, & Burnham EL (2016). Alveolar macrophage inflammatory mediator expression is elevated in the setting of alcohol use disorders Alcohol (Fayetteville, N.Y.), 50, 43–50. 10.1016/j.alcohol.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunsakin O, Hottor T, Mehta A, Lichtveld M, & McCaskill M (2016). Chronic Ethanol Exposure Effects on Vitamin D Levels Among Subjects with Alcohol Use Disorder. Environmental Health Insights, 10, 191–199. 10.4137/EHI.S40335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostaff MJ, Schäfer C, Courth L, Stebe SRD, Ott G, Stange EF, & Wehkamp J (2015). Chronic Heavy Alcohol Use is Associated with Upregulated Paneth Cell Antimicrobials in Gastric Mucosa. Clinical and Translational Gastroenterology, 6(7), e103–e103. 10.1038/ctg.2015.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis IL, Marrari M, Zeevi A, Duquesnoy RJ, Griffith BP, Hardesty RL, & Dauber JH (n.d.). HLA phenotype of lung lavage cells following heart-lung transplantation. The Journal of Heart Transplantation, 4(4), 422–425. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3939652 [PubMed] [Google Scholar]
- Park S-M, Lee JY, Hong S, Lee SH, Dimov IK, Lee H, … Lee LP (2016). Dual transcript and protein quantification in a massive single cell array. Lab on a Chip, 16(19), 3682–3688. 10.1039/c6lc00762g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaez A, Mitchell PO, Shah NS, Force SD, Elon L, Brown LAS, & Guidot DM (2015). The Role of Donor Chronic Alcohol Abuse in the Development of Primary Graft Dysfunction in Lung Transplant Recipients. The American Journal of the Medical Sciences, 349(2), 117–123. 10.1097/MAJ.0000000000000361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Samokhvalov AV, Neuman MG, Room R, Parry C, Lönnroth K, … Popova S (2009). The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health, 9(9). 10.1186/1471-2458-9-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds HY (1995). Pulmonary host defenses. Alcoholism, Clinical and Experimental Research, 19(1), 6–10. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7771664 [DOI] [PubMed] [Google Scholar]
- Rimland D (n.d.). Mechanisms of ethanol-induced defects of alveolar macrophage function. Alcoholism, Clinical and Experimental Research, 8(1), 73–76. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/6324608 [PubMed] [Google Scholar]
- Rio DC, Ares M, Hannon GJ, & Nilsen TW (2010). Purification of RNA Using TRIzol (TRI Reagent). Cold Spring Harbor Protocols, 2010(6), pdb.prot5439-pdb.prot5439. 10.1101/pdb.prot5439 [DOI] [PubMed] [Google Scholar]
- Samokhvalov AV, Irving HM, & Rehm J (2010). Alcohol consumption as a risk factor for pneumonia: a systematic review and meta-analysis. Epidemiology and Infection, 138(12), 1789–1795. 10.1017/S0950268810000774 [DOI] [PubMed] [Google Scholar]
- Sawyer JG, Martin NL, & Hancock RE (1988). Interaction of macrophage cationic proteins with the outer membrane of Pseudomonas aeruginosa. Infection and Immunity, 56(3), 693–698. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3125111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schögler A, Muster RJ, Kieninger E, Casaulta C, Tapparel C, Jung A, … Alves MP (2016). Vitamin D represses rhinovirus replication in cystic fibrosis cells by inducing LL-37. The European Respiratory Journal, 47(2), 520–530. 10.1183/13993003.00665-2015 [DOI] [PubMed] [Google Scholar]
- Seiler F, Bals R, & Beisswenger C (2016). Function of Antimicrobial Peptides in Lung Innate Immunity BT - Antimicrobial Peptides: Role in Human Health and Disease. In Harder J & Schröder J-M (Eds.) (pp. 33–52). Cham: Springer International Publishing; 10.1007/978-3-319-24199-9_3 [DOI] [Google Scholar]
- Sisson JH (1995). Ethanol stimulates apparent nitric oxide-dependent ciliary beat frequency in bovine airway epithelial cells. American Journal of Physiology-Lung Cellular and Molecular Physiology, 268(4), L596–L600. 10.1152/ajplung.1995.268.4.L596 [DOI] [PubMed] [Google Scholar]
- Stjärne Aspelund A, Hammarström H, Inghammar M, Larsson H, Hansson L, Christensson B, & Påhlman LI (2017). Heparin-binding protein, lysozyme and inflammatory cytokines in bronchoalveolar lavage fluid as diagnostic tools for pulmonary infection in lung transplanted patients. American Journal of Transplantation. 10.1111/ajt.14458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecle T, Tripathi S, & Hartshorn KL (2010). Review: Defensins and cathelicidins in lung immunity. Innate Immunity, 16(3), 151–159. 10.1177/1753425910365734 [DOI] [PubMed] [Google Scholar]
- Tiroke AH, Bewig B, & Haverich A (1999). Bronchoalveolar lavage in lung transplantation. State of the art. Clinical Transplantation, 13(2), 131–157. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10202611 [DOI] [PubMed] [Google Scholar]
- Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sørensen OE, Borregaard N, … Hiemstra PS (2003). The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. Journal of Immunology (Baltimore, Md. : 1950), 171(12), 6690–6696. 10.4049/JIMMUNOL.171.12.6690 [DOI] [PubMed] [Google Scholar]
- Viljanen P, Koski P, & Vaara M (1988). Effect of small cationic leukocyte peptides (defensins) on the permeability barrier of the outer membrane. Infection and Immunity, 56(9), 2324–2329. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3137167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wah Wellek Anne Frankenberger Marion Unterberger Pia Welsch Ulrich Bals Robert, J (2006). Antimicrobial peptides are present in immune and host defense cells of the human respiratory and gastroinstestinal tracts. Cell Tissue Res, 324, 449–456. 10.1007/s00441-005-0127-7 [DOI] [PubMed] [Google Scholar]
- Yeligar SM, Chen MM, Kovacs EJ, Sisson JH, Burnham EL, Ann L, & Brown S (2016). Alcohol and Lung Injury and Immunity HHS Public Access. Alcohol, 55, 51–59. 10.1016/j.alcohol.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]