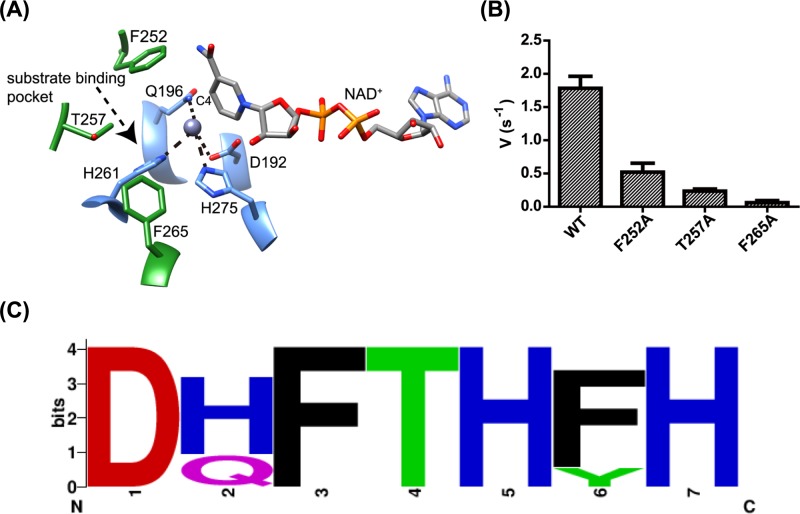
Figure 6. Active site structure and residues of BkTauF.
(A) Zoomed-in view of the active site of BkTauF. The substrate-interacting residues are displayed in green and labeled. Metal-coordinated residues are displayed in blue and labeled. Metal coordination is indicated by dashed lines. (B) Enzyme activities of BkTauF active site mutants. (C) Sequence logo showing the conservation of active site residues (D192, Q196, F252, T257, H261, F265 and H275) in close homologs of BkTauF in the UniRef cluster UniRef50_ A0A0J8DBY5.