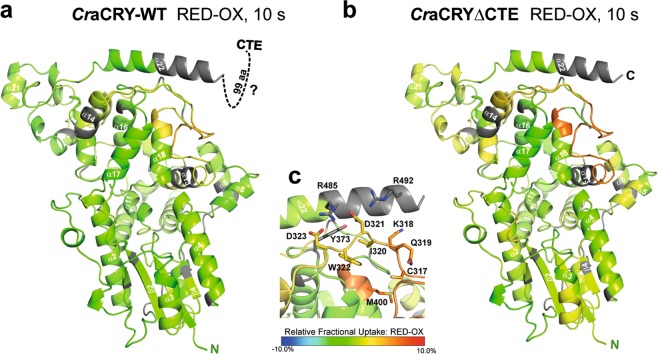
Figure 4.
Relative fractional deuterium uptake at 10 s of the reduced (FADH−) minus the oxidized (FADOX) states of (a) CraCRY-WT and (b) truncated CraCRY∆CTE, both are mapped onto the CraCRY∆CTE structure (PDB: 5ZM0). A highly significant difference can be observed in a loop region close to the C-terminal helix α22. c) The shown loop (300–326) presenting the distal tryptophan, W322, is part of the highly conserved tryptophan triad in photolyases and cryptochromes. W322 is flanked by the polar residues D323 and D321, which form a network of hydrogen bond interactions with the basic residues R485 and R492 from the C-terminal α22 helix. This charged network shields the distal electron donor of the electron transfer cascade, Y373, from exposure at the protein surface. The 320–326 loop exhibits a higher deuterium uptake in the reduced state, which correlates with higher flexibility. We suggest that the tight interaction between D323/D321 and R485/R492 gets disrupted upon formation of the Y373° radical, so that the α22 helix loses conformational restraint by interaction with the PHR.