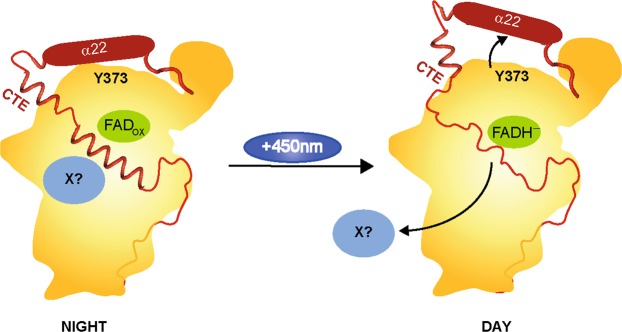
Figure 5.
Model of the structural movement in CraCRY from the fully oxidized to the hydroquinone state of the FAD based on the HDX data. In between, the neutral semiquinone state is formed (FADH°), but we have no structural information addressing this state. In the shown model, the C-terminal extension (CTE, red) is located over the protein surface and is partly structured. We propose that an unknown signaling partner X (light blue) can bind to CraCRY during the night, when it is located over the whole cell body. During the day, the hydroquinone state is forming (FADH−). Because of the radical pair formation [Y373° and FADH°] during photoreduction, the H-bond network between α22, D323 and D321 is disrupted which leads to a movement of the helix and therefore a change in the CTE. The structured region in the CTE gets unfolded and factor X is getting released.