Supplemental Digital Content is available in the text
Keywords: constipation, fecal microbiota transplantation, gut microbiota, Parkinson's disease, tremor
Abstract
Rationale:
Fecal microbiota transplantation (FMT) is recognized as an emerging treatment through reconstruction of gut microbiota. Parkinson's disease is a neurodegenerative disorder, which is accompanied by constipation. Here we first reported a patient with Parkinson's disease and constipation that were obviously relieved after FMT.
Patient concerns:
A 71-year-old male patient presented with 7 years of resting tremor, bradykinesia (first inflicted the upper limbs and subsequently spread to lower limbs), and intractable constipation (defecation needing more than 30 minutes).
Diagnoses:
Parkinson's disease for 7 years; constipation >3 years.
Interventions:
The patient had used madopar, pramipexole, and amantadine for anti-Parkinson and showed partially mitigation while laxative therapy for constipation failed. Finally FMT was performed.
Outcomes:
The patient successfully defecated within 5 minutes and maintained daily unobstructed defecation until the end of follow-up. The patient's tremor in legs almost disappeared at 1 week after FMT but recurred in the right lower extremity at 2 months after FMT.
Lessons:
Gut microbiota reconstruction may have therapeutic effects for Parkinson's disease patients, especially those who have gastrointestinal symptoms and limited treatment choices.
1. Introduction
Parkinson's disease (PD) is a progressive, chronic, neurodegenerative disorder, which always with gastrointestinal dysfunction. Patients with PD often demonstrate gastrointestinal symptoms, such as constipation, which precede the motor sign of PD.[1] The etiology of PD is not well understood but like to be the outcome of genetic factors and environmental effects, whereas the concept of “gut microbiota-brain axis” has been well established and its dysregulation may lead to neurological disease like PD.[2]
Accumulating evidence indicates a potential bidirectional interaction between gut microbiota and PD. A research showed gut microbiota was involved in motor deficits, microglia activation, and αSyn pathology which play important roles in the development of PD. Moreover, αSyn-overexpressing mice implanted with microbiota from PD-affected patients showed more obvious physical impairment compared to those with microbiota from healthy human donors.[3] Fecal microbiota transplantation (FMT), a technology by transplanting the gut microbiota of healthy people into the patients’ intestines, is recognized as an emerging treatment through reconstruction of gut microbiota, and is also considered as a treatment of Clostridium difficile infection, Crohn's disease, ulcerative colitis, constipations, as well as certain neurological diseases.[4,5]
Encouraged by numerous studies, we hypothesized that FMT may play a positive role in the treatment of PD. In this study, we reported the first case that used FMT to treat PD patient with constipation. The neurological and gastrointestinal symptoms (like tremor and constipation) of the patient were effectively reduced.
2. Case presentation
A 71-year-old male patient presented with 7 years of resting tremor and bradykinesia that first inflicted the upper limbs and subsequently spread to the right lower limb and left lower limb. In addition to the above symptoms, the patient has been plagued by constipation (defecation needing more than 30 minutes) for years. After diagnosis of PD in the local hospital, the patient started to use madopar, pramipexole, and amantadine for anti-Parkinson and showed partially mitigation while laxative therapy for constipation but showed very limited effect. According to the unified Parkinson's disease rating scale (UPDRS),[6] scores were given as follows: part II (difficulties in activities of daily living), 13/42; part III (motor examination), 46/142. Patient-assessment of constipation quality of life (PAC-QOL score),[7] and Wexner constipation score,[8] 18 and 16.
The patient was referred to Guangzhou First People's Hospital in October 2017 after former constipation treatment failed for FMT. We gained written informed consent from the patient. Routine examinations were completed to ensure the absence of contraindications of FMT. During colonoscopy, a transendoscopic enteral tubing (TET) tube (Nanjing FMT Medical Company, Nanjing, China) was inserted into the ileocecal junction through the endoscopy channel and fixed to the intestinal wall. The stool for FMT was obtained from a college student (26-year-old male) who also signed an informed consent form before donation. Stool donor was selected with a well-defined protocol adapted from the published literature.[9] The laboratory and clinical work flow were implemented according to the instruction of automatic purification system (GenFMTer; FMT Medical, Nanjing, China).[10] Then 200 ml of prepared fecal microbiota suspension was injected through the TET tube. This procedure was repeated for 3 times (once every day). No adverse reaction appeared during FMT. After the FMT treatment, the patient declared the obviously reduced tremor in both lower limbs and the easier and quicker defecation. During a follow-up of 3 months, we recorded the change of tremor, completed several surveys and sampled his fresh stool for 16s RNA microbiota analysis.
During the follow-up, the tremor in legs almost disappeared at 1 week after FMT treatment (see Video, Supplemental Video1-B). Though the resting tremor recurred in the right lower extremity at 2 months after FMT (see Video, Supplemental Video1-C), but its severity dropped compared with that of pre-FMT treatment (see Video, Supplemental Video1-A). In Table 1, the patient's UPDRS score began to decrease after FMT, and then decrease became significant at 1 week after treatment, but later, the score showed a trend increasing with time. PAC-QOL and Wexner constipation score suggested that the patient's constipation was significantly alleviated: the time of defecation was shortened from more than 30 minutes to 5 minutes. This effect continued until the end of follow-up. Coupled with the results of fecal microbiota analysis at different follow-up points, FMT could effectively raise the α-diversity of the gut microbiota (Fig. 1A and B). It was found by PCoA analysis that the patient's microbiota structure was similar to the donor's at 1 week after FMT, but the structural difference showed up with time went on (Fig. 1D). Using OTUs clustering and weighted unifrac tree analysis method (Fig. 1C and E), we found the abundance of Firmicutes increased while those of Proteobacteria and Bacteroidetes decreased after treatment, and the clustering of bacterial communities showed similarity between donor and patient after treatment. On genus level (Fig. 1F), the relative abundance of Lachnoclostridium, Dialister, Alistipes, and Unidentified-Ruminococcaceae increased after 1 week; that of Megamonas increased after 1 month, and that of Akkermansia and Faecalibacterium increased after 3 months.
Table 1.
Therapeutic evaluation of Parkinsonism and constipation.

Figure 1.
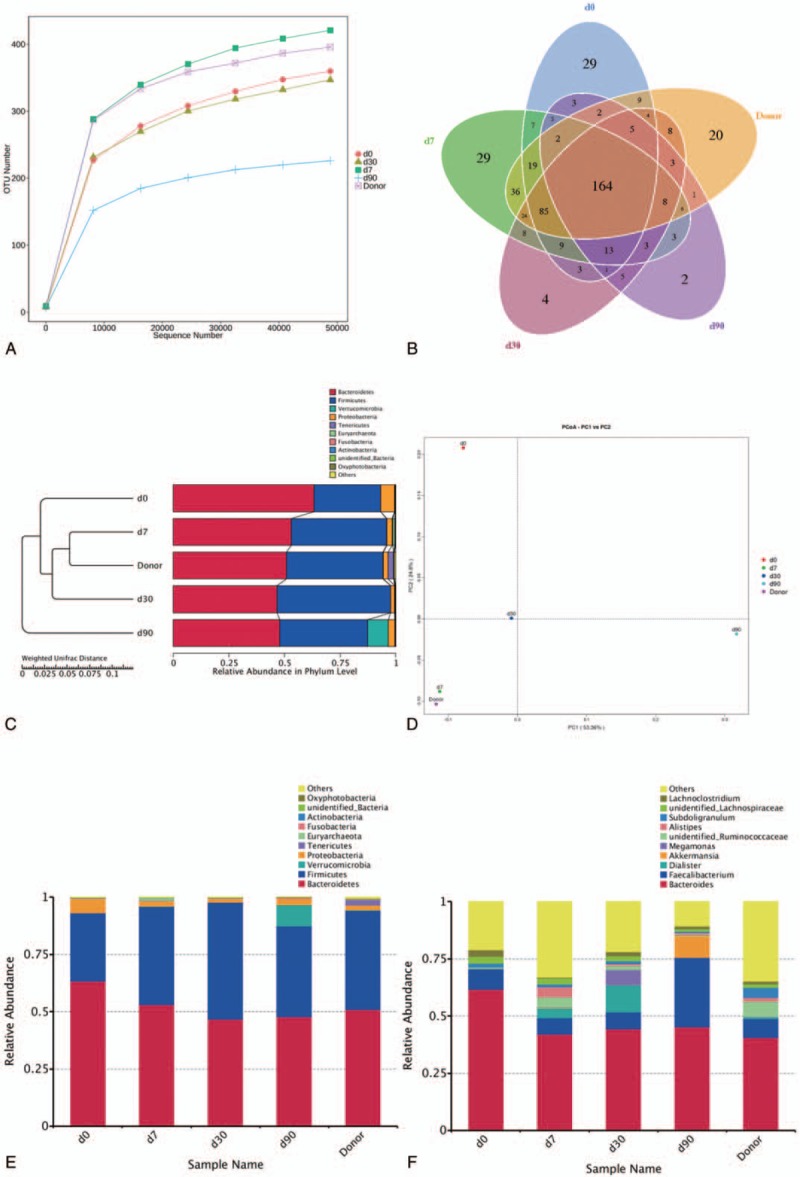
Microbiota analysis. Fecal microbial communities of the patient before FMT, at 1 wk after FMT, 1 mo after FMT and 3 mo after FMT. (A) Alpha diversity of observed species; (B) Venn diagram of shared and independent bacterial OTUs during different periods; (C) Weighted unifrac tree analysis, differences in the clustering of bacterial communities by the UPGMA; (D) PCoA; each dot represents 1 fecal sample; dot closeness indicates the similarity between bacterial communities. (E, F) Relative abundance of fecal bacterial taxa on phylum and genus levels, respectively. FMT = fecal microbiota transplantation, PCoA = principal co-ordinates analysis, UPGMA = unweighted pair group method with arithmetic mean.
3. Discussion
As a potential modulator of human biology, gut microbiota can directly or indirectly regulate brain neurochemistry through immune, metabolic, or endocrine mechanisms.[11] PD is a huge concern for the aging population. Mounting evidence proves that the gut microbiota is required for motor deficits and neuroinflammation in a model of PD.[3,12] In this case, the patient had suffered from serious tremor and constipation. Fortunately, the patient agreed to try FMT, and the outcomes were inspiring. Although this case was the first attempt to treat PD with FMT, the patient's constipation and tremor was effectively relieved. We also noticed that the patient's gut microbiota status was significantly associated with the severity of symptoms.
However, several problems needed to be settled. First, the patient’ tremor in limbs was decreased, but other Parkinson-related symptoms such as face and neck stiffness showed no change, even the patient's tremor disappeared at 1 week after treatment, indicating that the effect gradually diminished with time. Second, Scheperjans's[13] sequencing of 72 stool samples from PD patients and healthy controls found the abundance of Prevotella in PD patients decreased significantly. Keshavarzian et al[14] found the abundance of butyric acid-producing bacteria such as Blautia, Coprococcus, and Roseburia reduced in PD patients. But our report found a decline in the abundance of butyric acid-producing bacteria, but not of Prevotella. This difference should be illustrated with further research. Third, Megamonas, and Akkermansia genus increased after FMT treatment, and whether the enrichment of these bacteria was also associated with PD was unknown.
Braak et al[15,16] inferred that the gastrointestinal plexus was involved in the early onset of PD. Svensson et al[17] verified that cutting off the abdomen significantly reduced the prevalence of PD, which meant the intestinal activity may associate with PD. Treating PD with FMT has just taken a baby step, but it is enlightening enough to drive us farther into the magic of FMT in dealing with neurological diseases. In summary, our patient illustrates the efficacy of FMT in PD with constipation and its positive effect on clinical features. Alterations of pre- and post-FMT suggest potential for more targeted and specific FMT-based therapies in PD. This case report may open a new window to look into the mechanism of microbiota-gut-brain axis and the biotherapy for PD.
Acknowledgments
We thank the patient participated in this study and all staff in the Department of Gastroenterology.
Author contributions
HH and HX contributed equally to this work. HH involved in design of the study, recruitment of the patient, and drafting of the article; HX involved in statistical analysis and interpretation of the data, and drafting of the article; QL, JH performed the sample collection and DNA extraction; ML was the attending Neurologist and managed follow-up visits; HC and WT prepared fecal samples into filtrate for administration during the FMT procedure and revision of the article; YZ and YN designed and organized the study, interpretation of the data and revision of the article.
Data curation: Haoming Xu.
Investigation: Mengyan Li, Huiting Chen, Wenjuan Tang.
Methodology: Qingling Luo, Jie He.
Project administration: Huiting Chen, Wenjuan Tang, Yuqiang Nie, Yongjian Zhou.
Writing – original draft: Hongli Huang, Haoming Xu.
Writing – review and editing: Yuqiang Nie, Yongjian Zhou.
Yongjian Zhou orcid: 0000-0002-4036-4592.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: FMT = fecal microbiota transplantation, OUT = operational taxonomic units, PAC-QOL = patient-assessment of constipation quality of life, PCoA = principal co-ordinates analysis, PD = Parkinson's disease, TET = transendoscopic enteral tubing, UPDRS = unified Parkinson's disease rating scale.
HH and HX contributed equally to this work.
All data analyzed in this study are included in this report.
Committee of Guangzhou First People's Hospital approved the FMT study (K-2017-078-02). All methods were performed in accordance with the provisions of the Declaration of Helsinki of 1975. Before screening, we gained written informed consents from all the participants.
Written informed consent was obtained from the patient for publication of the case and any accompanying data.
This work was supported by Guangzhou General Science and Technology Project of Health and Family Planning (No. 20181A011007; No. 20191A011001); National Natural Science Foundation of China (NSFC 81871905); Natural Science Foundation of Guangdong Province (No. 2018A030313676) and Guangzhou Planned Project of Science and Technology (No. 201707010275, No. 201904010132).
All the authors have no conflict of interest relative to the research covered in this paper.
Supplemental Digital Content is available for this article.
References
- [1].Cersosimo MG, Raina GB, Pecci C, et al. Gastrointestinal manifestations in Parkinson's disease: prevalence and occurrence before motor symptoms. J Neurol 2013;260:1332–8. [DOI] [PubMed] [Google Scholar]
- [2].Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–12. [DOI] [PubMed] [Google Scholar]
- [3].Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell 2016;167:1469–80.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Reinisch W. Fecal microbiota transplantation in inflammatory bowel disease. Dig Dis (Basel, Switzerland) 2017;35:123–6. [DOI] [PubMed] [Google Scholar]
- [5].He Z, Cui B, Zhang T, et al. Fecal microbiota transplantation cured epilepsy in a case with Crohn's disease: the first report. World J Gastroenterol 2017;23:3565–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rodriguez-Molinero A, Sama A, Perez-Lopez C, et al. Analysis of correlation between an accelerometer-based algorithm for detecting Parkinsonian gait and UPDRS subscales. Front Neurol 2017;8:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Duncan PI, Enters-Weijnen CF, Emami N, et al. Short-term daily intake of polydextrose fiber does not shorten intestinal transit time in constipated adults: a randomized controlled trial. Nutrients 2018;10:E920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Agachan F, Chen T, Pfeifer J, et al. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum 1996;39:681–5. [DOI] [PubMed] [Google Scholar]
- [9].Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015;149:102–9.e6. [DOI] [PubMed] [Google Scholar]
- [10].Cui B, Li P, Xu L, et al. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitis. J Transl Med 2015;13:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yoo BB, Mazmanian SK. The enteric network: interactions between the immune and nervous systems of the gut. Immunity 2017;46:910–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pagano G, Ferrara N, Brooks DJ, et al. Age at onset and Parkinson disease phenotype. Neurology 2016;86:1400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Scheperjans F, Aho V, Pereira PA, et al. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord 2015;30:350–8. [DOI] [PubMed] [Google Scholar]
- [14].Keshavarzian A, Green SJ, Engen PA, et al. Colonic bacterial composition in Parkinson's disease. Mov Disord 2015;30:1351–60. [DOI] [PubMed] [Google Scholar]
- [15].Braak H, Ghebremedhin E, Rub U, et al. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res 2004;318:121–34. [DOI] [PubMed] [Google Scholar]
- [16].Braak H, de Vos RA, Bohl J, et al. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett 2006;396:67–72. [DOI] [PubMed] [Google Scholar]
- [17].Svensson E, Horvath-Puho E, Thomsen RW, et al. Vagotomy and subsequent risk of Parkinson's disease. Ann Neurol 2015;78:522–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


