Figure 3.
Time Course of Minimal Midzone Bundle Formation
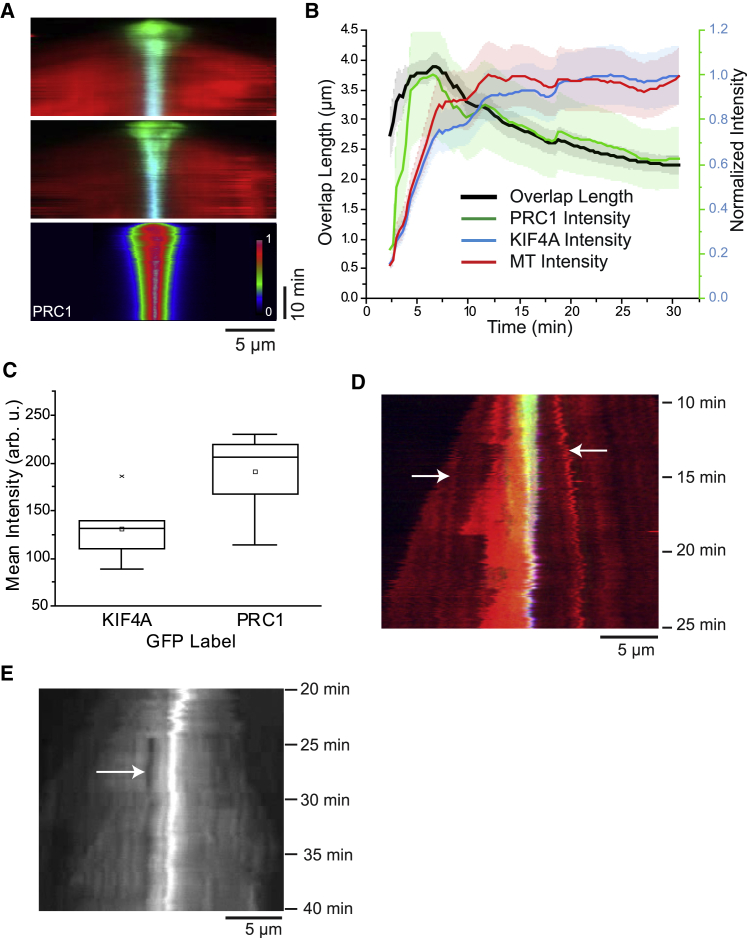
(A) (Top) Kymographs showing the time course of the formation of two antiparallel bundles in the presence of 20-nM PRC1-Alexa546 (green), 50-nM KIF4A-mBFP (blue), and 12.5-μM Alexa647-tubulin (red), imaged by TIRF microscopy (condition as in Figures 1C and 1D). (Bottom) Average normalized PRC1 intensity (n = 14) is shown.
(B) Mean overlap length and normalized mean total fluorescence intensity measured in the overlap region of minimal midzone bundles for Alexa647-tubulin, PRC1-Alexa546, and KIF4A-mBFP plotted as a function of time (n = 17); protein concentrations as in (A). The shaded areas show the SE.
(C) Boxplots showing the mean intensity of KIF4A and PRC1 in the overlap region at the endpoint of overlap formation (∼30 min; n = 8); protein concentrations as in (A). To enable a comparison of absolute final amounts of the proteins, two sets of experiments were done with either the KIF4A or PRC1 labeled with GFP, showing a 1.5- to 2-fold excess of PRC1 over KIF4A.
(D) Kymograph showing the tubulin fluorescence of an antiparallel bundle consisting of 3 microtubules soon after nucleation. The white arrows indicate speckles of higher tubulin labeling density, which show slow (∼3 nm/s) antiparallel sliding. Time is in minutes after initiating microtubule nucleation. Protein concentrations are as in Figure 2A.
(E) Kymograph showing the tubulin fluorescence of a minimal midzone bundle formed in the presence of 5-nM PRC1-Alexa546, 50-nM KIF4A-mBFP, and 12.5-μM Alexa647-tubulin. A bleach mark was placed on the bundle outside of the central antiparallel microtubule overlap ∼25 min after initiating nucleation. The distance between the bleach mark and the overlap remained constant. The temperature was 30°C.
See also Figures S2 and S3.