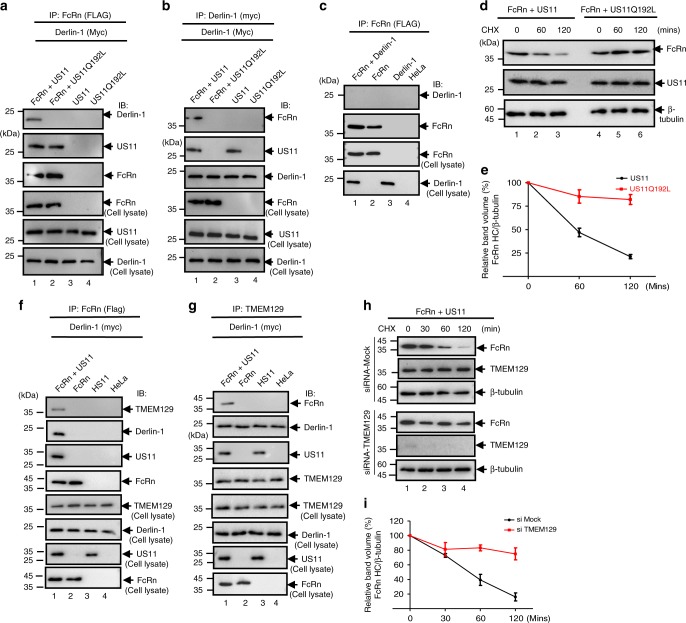
Fig. 4.
US11 recruits FcRn to Derlin-1 and TMEM129 protein complex. a–c US11 recruits FcRn to the Derlin-1 complex. US11Q192L represents a mutant US11 in which Q192 is replaced with leucine. Stable HeLaFcRn, HeLaFcRn+US11, HeLaFcRn+US11 Q192L, HeLaUS11, and HeLaUS11 Q192L cell lines were transfected with a plasmid encoding myc-tagged Derlin-1. Forty-eight hours after transfection, the cell lysates (0.5 mg) were immunoprecipitated with mAb anti-FLAG for FcRn (a, c) or anti-myc for Derlin-1 (b). Non-transfected HeLaFcRn or HeLa cells were used as a negative control. d, e FcRn in mutant US11Q192L transfected cells resists degradation. HeLaFcRn+US11 and HeLaFcRn+US11* cells were treated with CHX (100 μg/ml) for the indicated time (d). Cells were lysed after CHX treatment and cell lysates (20 μg) were subject to western blotting with corresponding Abs as indicated. The level of remaining FcRn (e) at different time points was quantified as the percentage of the β-tubulin level. f, g US11 recruits FcRn to TMEM129 complex. HeLaFcRn+US11 (lane 1), HeLaFcRn (lane 2), HeLaUS11 (lane 3), and HeLa cells were transfected with Derlin-1 plasmid. Forty-eight hours later, the cell lysates were immunoprecipitated by mAb anti-FLAG for FcRn (f) or anti-TMEM129 Ab (g). h, i TMEM129 is involved in US11-mediated FcRn degradation. The HeLaFcRn+US11 cells were transfected with 20 nM TMEM129 siRNA oligomers (h, bottom). Forty-eight hours later, cells were then treated with CHX (100 μg/ml) for the indicated time. The cells were lysed after CHX treatment and cell lysates (20 μg) were subjected to western blotting with Abs as indicated. The level of remaining FcRn (i) in TMEM129 siRNA-treated cells (red) or mock-treated cells (black) at different time points was quantified as the percentage of the β-tubulin level. All immunoprecipitates or cell lysates (20 μg) as controls were subject to western blotting with corresponding Abs as indicated (a, b, c, f, and g). The percentage of time point 0 (min) is assigned a value of 100% and the values from other time points are normalized to this value (e, i). Error bars: mean ± SEM of three independent repeats