Fig. 5.
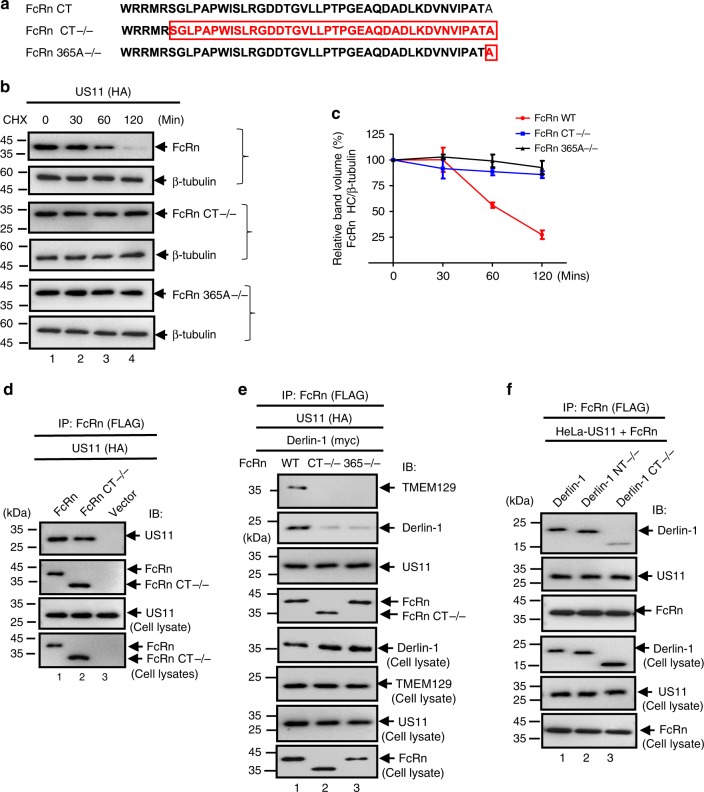
The cytoplasmic tail of FcRn contributes to US11-mediated degradation. a Depiction of tailless FcRn (CT−/−) and FcRn HC deleting alanine residue 365 (365A−/−) in the cytoplasmic tail. Letter(s) in red represent(s) the deleted amino acid(s). b, c Tailless FcRn or FcRn 365A−/− resists degradation in the presence of US11. HeLaUS11 (b, top), HeLaUS11+FcRn CT−/− (b, middle), or HeLaUS11+FcRn365−/− cells (b, bottom) were treated with CHX (100 μg/ml) and chased for the indicated time in the absence of proteasome inhibitors. The cells were lysed after CHX treatment and cell lysates (20 μg) were subject to western blotting with Abs as indicated. The level of wild-type FcRn (c, red), tailless FcRn (c, blue), and mutant FcRn 365A−/− (c, black) in HeLaUS11 cells at different time points was quantified as the percentage of the β-tubulin level. The percentage of time point 0 (min) is assigned a value of 100% and the values from other time points are normalized to this value (c). Error bars represented mean ± SEM of three independent repeats. d The US11 interacts with tailless FcRn protein. The cell lysates from HeLaFcRn+US11 (lane 1), HeLaUS11+FcRn CT−/− (lane 2), and HeLacontrol (lane 3) were immunoprecipitated by anti-FLAG Ab for FcRn. The US11 molecules that coprecipitate in the complex are indicated. e The cytoplasmic tail of FcRn is required for tightly binding to Derlin-1 in the presence of US11. HeLaFcRn+US11 (lane 1), HeLaUS11+FcRn CT−/− (lane 2), and HeLaUS11+FcRn 365A−/− (lane 3) cells were transfected with Derlin-1 plasmid. Forty-eight hours later, cell lysates were immunoprecipitated with anti-FLAG Ab to detect FcRn. f The C-terminus of Derlin-1 is required for tightly binding to FcRn in the presence of US11. The HeLaUS11+FcRn stable cells were transfected with a plasmid encoding Myc-tagged Derlin-1 (WT), Derlin-1 lacking its N-terminus (NT−/−) or C-terminus (CT−/−), respectively. Forty-eight hours after transfection, the cell lysates (0.5 mg) were immunoprecipitated with rabbit anti-FLAG Ab. All immunoprecipitates or cell lysates (20 μg) as internal controls were subject to western blotting with corresponding Abs as indicated (d–f)