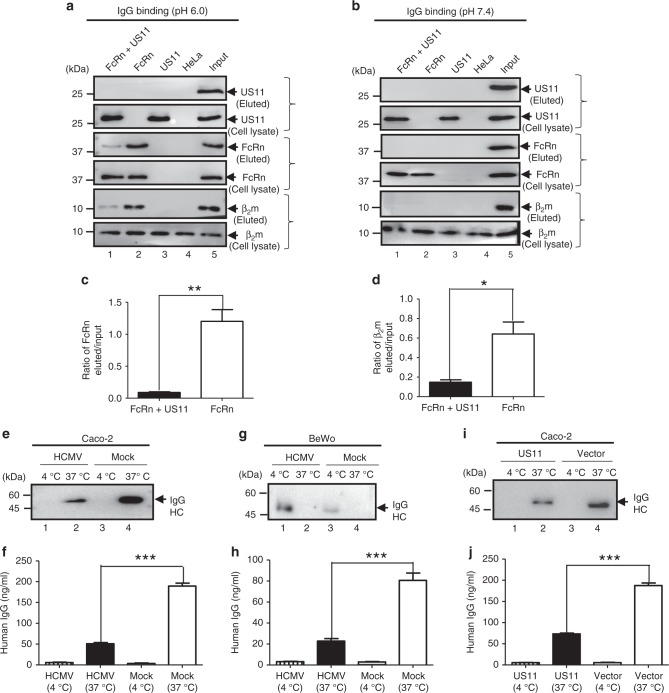
Fig. 8.
HCMV and US11 reduce FcRn-mediated IgG transcytosis in epithelial monolayers. a–d The presence of US11 reduces FcRn binding to IgG. HeLa transfectants were lysed in pH 6.0 (a) or pH 7.4 (b) condition. Approximately 0.5 mg of soluble proteins were incubated with human IgG-Sepharose at 4 °C. Eluted proteins were subjected to western blotting analysis with corresponding Abs as indicated. Cell lysates were blotted as control. The amount of eluted FcRn (c) or β2m protein from HeLaFcRn and HeLaFcRn+US11 cells was compared by the ratio of the band density of eluted protein to that of input protein. The density of protein bands was quantified by the Image Lab 5.2 software. e–h Caco-2 cells (2 × 104/well) or BeWo cells (105/well) were grown in 0.4 μm transwell for 8 to 10 days (Caco-2) or for 4 days (BeWo) to allow differentiation. When the transepithelial resistance r reached above 600 (Caco-2) or 400 (BeWo) ohms/cm2, cells were infected at the basolateral surface with HCMV (MOI 5) for 1 h. After washing, cells were incubated for 48 h. Infected or mock-infected cells were loaded at the apical surface with IgG (lanes 1–4) (0.5 mg/ml for Caco-2 or 0.25 mg/ml for BeWo) at 37 °C or 4 °C, respectively. Medium was collected from the basolateral compartment 2 h later and subjected to western blot (e, g) or ELISA (f, h) analysis. i, j Caco-2 cells transfected with either pEF6 or pEF6-US11 plasmid were grown on transwell inserts. The cells were incubated for 1 h at 37 °C or 4 °C, then IgG (0.5 mg/ml) was added to the apical surface and further incubated for 2 h to allow transcytosis. Medium from the basolateral compartment was collected and IgG content was measured by western blot (i) or ELISA (j). *P < 0.05, **P < 0.01, and ***P < 0.001 by Student’s t-test. Error bars represented mean ± SEM of three independent repeats