Figure 7.
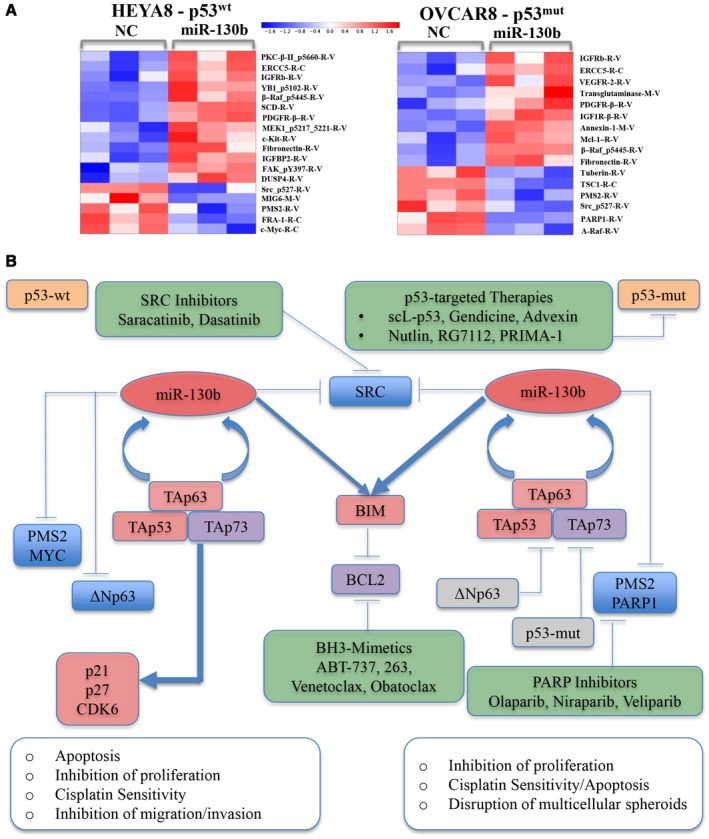
The proteomic footprint of microRNA 130b (miR‐130b) is illustrated in HEYA8 and OVCAR8 xenografts, a model for critical effectors downstream of miR‐130b, and a framework for the therapeutic development of miR‐130b for treating ovarian and other cancers. (A) Heat maps of proteins that are downregulated (blue) and upregulated (red) by >1.25‐fold upon treatment of HEYA8 and OVCAR8 cells with miR‐130b or negative control (NC) are depicted after reverse phase protein array analysis. (B) Genes that were upregulated (red), downregulated (blue), and did not change in response to miR‐130b (gray) treatment are depicted along with genes that have been established as connected (but were not measured) in the current study (purple). Therapeutic agents and/or strategies are indicated that are currently used or are being clinically tested in phase 1 or 2 trials for treating ovarian cancer (OVCA) (green) that target 1 or several direct or indirect downstream targets of miR‐130b. ABT‐737 indicates a small‐molecule B‐cell lymphoma (Bcl‐2) and Bcl‐xL drug; BCL2, B‐cell lymphoma; BIM, B‐cell lymphoma 2‐like protein 11;p53mut, mutant p53; p53wt, wild‐type p53; PARP, poly‐adenosine diphosphatase polymerase; PMS2, postmeiotic segregation increased, Saccharomyces cerevisiae, 2; PRIMA‐1, proline‐rich membrane anchor 1; scL‐p53, scL‐p53 tumor‐targeted nanocomplex; SRC, proto‐oncogene tyrosine protein kinase Src; TAp53, transactivation domain of tumor protein p53; TAp63, transactivation domain of tumor protein p63; TAp73, transactivation domain of tumor protein p73; ΔNp63, N‐terminally truncated (ΔN) isoform of the p63 protein.
