Figure 2.
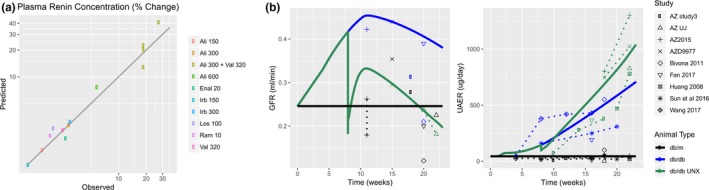
Case study 1. (a) When multiple high‐quality literature studies are available, each study should be included and used in parameter estimation to avoid bias and overfitting to a single study. Each observed value represents the mean plasma renin concentration (PRC) response from a single drug/dose combination for a particular study of drugs targeting the renin‐angiotensin‐aldosterone system (RAAS) pathway. In a model of the RAAS pathway, parameters defining negative feedback on renin secretion were estimated by simultaneously fitting the response to all studies rather than fitting to an individual study. (b) When a single study measuring all timepoints and variables of interest is not available, it is often possible to combine data from multiple studies to generate a composite time course. The time courses of glomerular filtration rate (GFR; left), urinary albumin excretion rate (UAER; right), and other variables (not shown) in db/db and db/db uninephrectomized (UNX) mice were assembled from multiple studies that measured these variables at some but not all times of interest. A quantitative systems pharmacology (QSP) model of diabetic kidney disease progression was calibrated by fitting these composite time courses (points and dashed lines = data; solid lines = model). AZ, AstraZeneca; Ali, aliskiren; Val, valsartan; Irb, irbesartan; Los, losartan; Enal, enalapril; Ram, ramipril db/db; db/m ‐ are not abbreviations. they are standard names for those mouse models
