Figure 6.
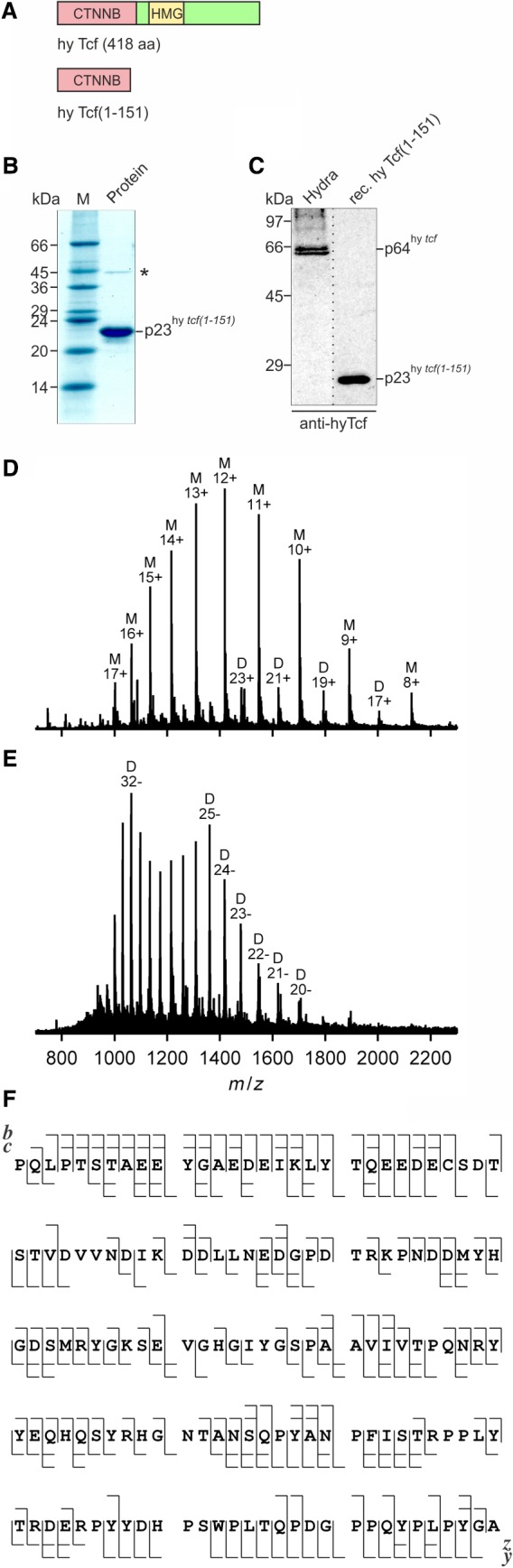
Analysis of the Hydra Tcf recombinant protein. (A) Schematic depiction of the Hydra (hy) Tcf protein product (GenBank accession no. XP_002159974). The positions of the high mobility group (HMG) representing the DNA binding domain, and the β‐Catenin binding site (CTNNB) are indicated. The amino‐terminal segment of Hydra Tcf [hy Tcf(1‐151)] was expressed in Escherichia coli and purified. (B) SDS/PAGE (12.5%, wt/vol) of 5 μg (Coomassie brilliant blue staining) purified recombinant Hydra Tcf(1‐151) p23. The upper faint band marked with an asterisk represents a cross‐linked dimer generated from p23hy tcf(1‐151) (see below). (C) Immunoblot analysis using 10 μg of cell extract from Hydra, and 5 ng of recombinant hy Tcf(1‐151). Proteins were resolved by SDS/PAGE (10%, wt/vol) and detected using an antiserum directed against recombinant hy Tcf(1‐151). The dotted line marks the splicing site in the blot image, from which one lane has been removed. (D–F) MS of recombinant hy Tcf(1‐151). The ESI‐MS of hy Tcf(1‐151) with a 7 Tesla Fourier transform ion cyclotron resonance (FT‐ICR) instrument (Bruker, Vienna, Austria) gives a mass value for the most abundant isotopic peak of 17 014.868 ± 0.006 Da (theoretical mass without initiating methionine: 17 014.874 Da; error 0.4 p.p.m.) using polyethylene glycol 1000 as calibrant. (D) ESI mass spectrum of hy Tcf‐NT in positive ion mode showing mostly (~ 95%) monomeric protein (M) but also protein dimers whose mass values (measured mass for the most abundant isotopic peak 34 027.727 Da) indicate covalent dimerization by formation of an intermolecular disulfide bond (theoretical mass for the most abundant isotopic peak 34 027.732 Da). (E) ESI of hy Tcf‐NT in negative ion mode gives predominantly (> 80%) dimer ions from intermolecular disulfide bond formation. (F) Fragment ion maps for the Hydra Tcf(1‐151) p23 protein showing 85% sequence coverage.
