Figure 1.
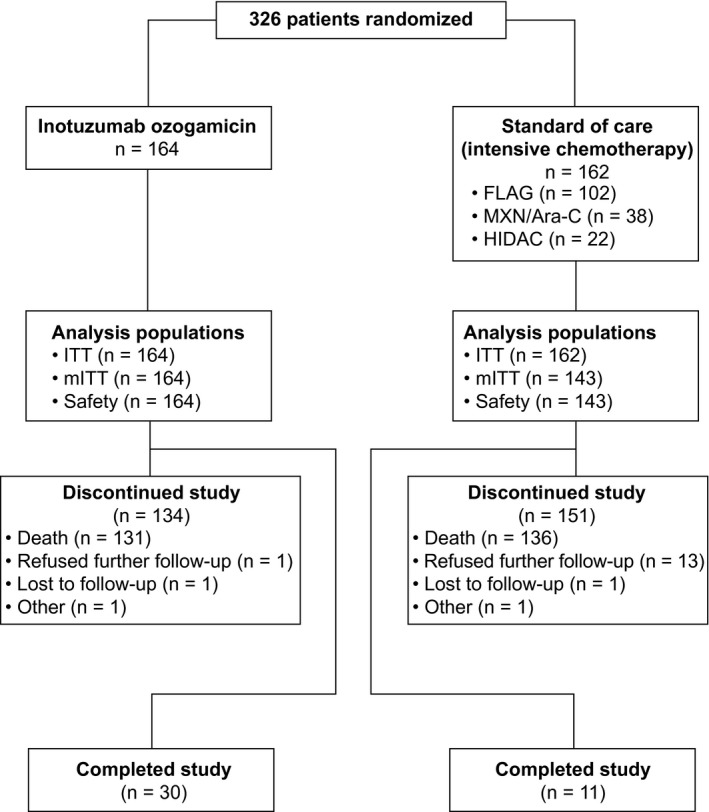
Trial profile. Completing the study was defined as completing follow‐up for 5 years from the individual patient’s randomization or for 2 years from randomization of the last patient (January 4, 2015), whichever occurred first. On the date of the last visit by the last patient to be randomized (January 4, 2017), all patients discontinued the study. In the InO arm, patients received InO intravenously at 1.8 mg/m2 per cycle, 0.8 mg/m2 on day 1 and 0.5 mg/m2 on days 8 and 15 of a 21‐ to 28‐day cycle, for a maximum of 6 cycles. Patients who achieved CR/CRi had their InO dose adjusted to 1.5 mg/m2 per cycle, with 0.5 mg/m2 administered on days 1, 8, and 15. Patients in the standard‐of‐care arm received a regimen of the investigator’s choice: 1) FLAG for up to four 28‐day cycles, which consisted of cytarabine at 2.0 g/m2/d on days 1 to 6, fludarabine at 30 mg/m2/d on days 2 to 6, and granulocyte colony‐stimulating factor at 5 μg/kg/d (or at the standard dose of the institute); 2) MXN/Ara‐C for up to four 15‐ to 20‐day cycles, which consisted of cytarabine at 200 mg/m2/d on days 1 to 7 and mitoxantrone at 12 mg/m2/d on days 1 to 3 (with dose reductions to 8 mg/m2 allowed on the basis of age, coexisting conditions, and previous anthracycline exposure); or 3) HIDAC for up to two 12‐dose cycles at 3.0 g/m2 every 12 hours (the dose could be reduced up to 1.5 g/m2 for patients aged 55 years or older and was reduced to 1.5 g/m2 for patients older than 60 years). CR indicates complete remission; CRi, complete remission with incomplete hematologic recovery; FLAG, fludarabine, cytarabine, and granulocyte colony‐stimulating factor; HIDAC, high‐dose cytarabine; InO, inotuzumab ozogamicin; ITT, intent‐to‐treat; mITT, modified intent‐to‐treat; MXN/Ara‐C, mitoxantrone and cytarabine.
