Abstract
Aims
To investigate whether the benefits of switching to insulin degludec observed in the European retrospective chart review study EU‐TREAT were dependent on the previous basal insulin used.
Methods
People with Type 1 or Type 2 diabetes were switched to insulin degludec from other basal insulins ≥6 months before data collection. Participants were stratified into three groups based on their previous basal insulin: insulin glargine 100 units/ml (Type 1: n=888; Type 2: n=259); insulin detemir (Type 1: n=726; Type 2: n=415); and neutral protamine Hagedorn (Type 1: n=53; Type 2: n=95). Their glycaemic control and hypoglycaemia incidence at 6 and 12 months post‐switch vs pre‐switch was then evaluated.
Results
Significant HbA1c reductions were achieved in all previous basal insulin groups for participants with Type 1 diabetes [insulin glargine 100 units/ml: −2.08 mmol/mol (−0.19%); insulin detemir: −2.40 mmol/mol (−0.22%)] and those with Type 2 diabetes [insulin glargine 100 units/ml: −5.90 mmol/mol (–0.54%); insulin detemir: −6.01 mmol/mol (−0.55%); neutral protamine Hagedorn: −2.73 mmol/mol (−0.25%)] at 6 months, except for the relatively small neutral protamine Hagedorn group in those with Type 1 diabetes [−1.75 mmol/mol (−0.16%)], where statistical significance was not reached. At 6 months in the Type 1 diabetes group, switching to insulin degludec from insulin glargine 100 units/ml resulted in significantly lower hypoglycaemia rates across all hypoglycaemia categories; for the insulin detemir group, this significance was also observed for severe and nocturnal non‐severe hypoglycaemia, while the low number of people in the neutral protamine Hagedorn group resulted in nonsignificant reductions in hypoglycaemia rates. At 6 months in the people with Type 2 diabetes, switching to insulin degludec resulted in significantly lower rates of hypoglycaemia across all categories for all groups. Similar outcomes were observed at 12 months.
Conclusions
Switching to insulin degludec from other basal insulins can improve glycaemic control and/or reduce hypoglycaemia risk in people with diabetes (although there was a nonsignificant reduction in HbA1c and hypoglycaemia rates for the neutral protamine Hagedorn group in Type 1 diabetes) under routine care.
What's new?
The EU‐TREAT study previously demonstrated that switching to insulin degludec from other basal insulins improved glycaemic control and significantly lowered the risk of hypoglycaemia in people with Type 1 or Type 2 diabetes.
In the present study, people from EU‐TREAT were stratified into three groups, based on type of previous basal insulin used.
Results showed that people who switched to insulin degludec from glargine U100 or detemir benefitted from improved glycaemic control and a lowered risk of hypoglycaemia at lower or similar insulin doses.
This study indicates that insulin degludec can offer improved glycaemic control and reduced hypoglycaemia risk, irrespective of previous basal insulin therapy.
What's new?
The EU‐TREAT study previously demonstrated that switching to insulin degludec from other basal insulins improved glycaemic control and significantly lowered the risk of hypoglycaemia in people with Type 1 or Type 2 diabetes.
In the present study, people from EU‐TREAT were stratified into three groups, based on type of previous basal insulin used.
Results showed that people who switched to insulin degludec from glargine U100 or detemir benefitted from improved glycaemic control and a lowered risk of hypoglycaemia at lower or similar insulin doses.
This study indicates that insulin degludec can offer improved glycaemic control and reduced hypoglycaemia risk, irrespective of previous basal insulin therapy.
Introduction
Insulin treatment is the most efficacious therapy option for reducing HbA1c levels in people with diabetes; however, tight glycaemic control is typically accompanied by an increased risk of hypoglycaemia 1, which has therefore been a barrier to insulin initiation and intensification 2. Insulin products that carry a relatively reduced risk of hypoglycaemia without sacrificing glycaemic control are therefore desirable 2, 3.
Insulin degludec (degludec) is a basal insulin with a duration of action exceeding 42 h at clinically relevant doses and a lower day‐to‐day variability in blood glucose‐lowering profile, compared with insulin glargine 100 units/mL (glargine U100) and insulin glargine 300 units/mL (glargine U300) 4, 5, 6. Randomized controlled trials in adults have indicated that degludec is associated with a reduced risk of hypoglycaemia compared with other long‐acting insulin analogues at equivalent levels of glycaemic control 7, 8, 9, 10, 11.
In addition, a real‐world study, the European, multicentre, retrospective, non‐interventional chart review study [European Tresiba Audit (EU‐TREAT); NCT02662114, n=2550], has evaluated the clinical effectiveness of switching to degludec in insulin‐treated adults with Type 1 (n=1717) or Type 2 diabetes (n=833) under routine clinical care 12. The study showed that switching to degludec from other basal insulins improved glycaemic control and significantly reduced the risk of hypoglycaemia with lower or similar insulin dose requirements 12. It is not known, however, whether switching to degludec from different previous basal insulins produces heterogeneous responses with regard to glycaemic control and risk of hypoglycaemia. It is therefore of interest to stratify participants from EU‐TREAT, based on the type of previous basal insulin received, to investigate whether the observed clinical benefits of switching to degludec are dependent on the type of previous basal insulin therapy in people with Type 1 or Type 2 diabetes.
Methods
EU‐TREAT study overview
The present secondary analysis used data from EU‐TREAT, a European, multicentre, retrospective, non‐interventional chart review study. EU‐TREAT investigated the clinical effectiveness of switching to degludec (100 units/mL or 200 units/mL; Novo Nordisk, Bagsværd, Denmark) ± prandial insulin from any basal insulin, in insulin‐treated adults with either Type 1 or Type 2 diabetes (± oral antidiabetic drugs in Type 2 diabetes), under conditions that reflected routine clinical care in multiple centres across Europe. Previous basal insulin regimens included glargine U100, insulin detemir (IDet), neutral protamine Hagedorn (NPH), glargine U300 and other basal insulins. Baseline was defined as the most recent recording during the 3‐month period prior to degludec initiation. Outcome data, for both pre‐ and post‐switch, were collected at 6±3 and 12±3 months during the periods before and after the switch. The source data were monitored monthly off‐site; participants’ chart data, taken from participants’ records, were entered in the electronic data capture system. The ad hoc site management calls and/or e‐mails were in place, dependent on site issues and follow‐up activities. On‐site monitoring visits at selected sites were also in place if required and approved by the sponsor and project manager. The detailed study design and methodology for EU‐TREAT have been described previously 12.
A list of the study sites was identified by Novo Nordisk local affiliates. From this list, a number of study sites were randomly selected by the Contract Research Organisation (ICON plc, Dublin, Ireland), which subsequently sent the blinded questionnaires to the study sites to confirm their interest and eligibility. Confirmed investigators invited eligible participants in a consecutive manner to participate and sign the study consent form.
The inclusion criteria specified people (aged ≥18 years at the time of degludec initiation) with Type 1 or Type 2 diabetes, treated with insulin for >12 months, switched to degludec [± prandial insulin (±oral antidiabetic drugs in Type 2 diabetes)] from any other basal insulin (± prandial insulin [±oral antidiabetic drugs in Type 2 diabetes]) at least 6 months prior to data collection, and treated with basal insulin for at least 6 months before switching. The following exclusion criteria were applied: previous participation in this study, or a diabetes clinical trial, or receipt of any investigational medicinal product up to 12 months before or any time after the initiation of degludec; current participation in another non‐interventional study on degludec; people with diabetes treated by continuous subcutaneous insulin infusion or premixed insulin in the 6 months prior to receiving degludec.
The primary outcome was change in HbA1c after 6 months, compared with the last value on previous basal insulin before switch. Other outcomes included change in HbA1c at 12 months, rates of overall, overall non‐severe, severe and nocturnal non‐severe hypoglycaemic events, and changes in total, basal and prandial insulin dose, 6 and 12 months post‐ vs pre‐switch. Six‐month data were available for all people with diabetes included in this study; 12‐month data were available for 76% of those with Type 1 diabetes and 72% of those with Type 2 diabetes. Overall hypoglycaemic events were those recorded by the physician/nurse in the participants’ charts. Severe hypoglycaemia was defined as an episode requiring the assistance of another person to actively administer carbohydrate, glucagon or other corrective actions. Overall non‐severe hypoglycaemia included any non‐severe hypoglycaemic events. Nocturnal non‐severe hypoglycaemia was defined as any non‐severe event in which the words ‘nocturnal’ or ‘night’ (or their equivalent in the local language) were present in the participants’ records.
The study protocols were approved according to local regulations by appropriate health authorities and by institutional review boards at all participating institutions, and conducted in accordance with the Declaration of Helsinki 13. Written informed consent from all participants was obtained before enrolment.
Post hoc analysis
In the present study, people with diabetes from the EU‐TREAT study were stratified into three groups, glargine U100, IDet and NPH, based on their type of previous basal insulin before switching to degludec, as these groups included a sufficient number of participants for statistical analyses. Participants treated with other insulins (Type 1 diabetes: n=27; Type 2 diabetes: n=39) 12 or with missing data on previous basal insulins (Type 1 diabetes: n=23; Type 2 diabetes: n=25) 12 before switching to degludec were excluded from the present study. The clinical outcomes described above are reported in the present study.
Statistical analyses
Baseline characteristics and demographics are reported using descriptive statistics, with mean (sd) or percentage as appropriate.
Change in HbA1c, and ratios of total, basal and prandial insulin doses (log[dose]) at 6 and 12 months post‐switch vs pre‐switch were analysed using a multivariate analysis of covariance, adjusting for age, BMI, gender, diabetes duration, duration of insulin therapy and type of basal injections. The normality of residuals for the primary endpoint was assessed to confirm that the used model was satisfactory with regard to model fit.
The number and rates of hypoglycaemic events were analysed using a negative binomial regression estimator including a variable to capture differential exposure across participants and a variable to capture the effects of post‐ vs pre‐switch. The paired data were addressed by controlling for subjects (REPEATED statement in SAS GENMOD) 14 and estimates, adjusted using general estimating equations, were provided. Comparisons of hypoglycaemic event rates were based on similar time frames, i.e. 0 to +6 vs −6 to 0 months, or 0 to +12 vs −12 to 0 months post‐ and pre‐switch, respectively. All analyses included participants with relevant data at both the pre‐ and post‐switch time points. In addition, the effect of previous basal insulin type on the 6‐month comparisons in overall hypoglycaemia was tested using the Wald test. Because of the low number of participants, the Wald test could not be performed for the NPH group; therefore, for the present analysis, the previous insulin type, IDet or glargine U100, was included as covariate in the model. The threshold for significance was P<0.05.
Results
Baseline characteristics
Baseline characteristics of stratified groups with Type 1 diabetes (overall, n=1717; glargine U100, n=888; IDet, n=726; NPH, n=53) or Type 2 diabetes (overall, n=833; glargine U100, n=259; IDet, n=415; NPH, n=95) are presented in Table 1.
Table 1.
Baseline characteristics of people who switched to degludec from other basal insulins, stratified according to their previous basal insulin
| Baseline characteristic | Type 1 diabetes | Type 2 diabetes | ||||||
|---|---|---|---|---|---|---|---|---|
| Glargine U100 | IDet | NPH | Overall 12 | Glargine U100 | IDet | NPH | Overall 12 | |
| Full analysis set, n | 888 | 726 | 53 | 1717 | 259 | 415 | 95 | 833 |
| Age, years | 46.5 (15.4) | 49.3 (15.9) | 47.0 (13.3) | 47.7 (15.6)* | 63.9 (10.6) | 65.1 (10.5) | 65.5 (10.1) | 64.6 (10.5)* |
| Female/male, % | 45.0/55.0 | 45.7/54.3 | 49.1/50.9 | 45.7/54.3 | 40.5/59.5 | 43.6/56.4 | 44.2/55.8 | 42.4/57.6 |
| Weight, kg | 75.8 (16.3) | 78.8 (16.4) | 81.8 (17.7) | 77.4 (16.4) | 93.2 (20.6) | 97.7 (20.4) | 103.3 (22.9) | 97.2 (21.0) |
| BMI, kg/m2 | 25.9 (4.6) | 26.6 (4.9) | 27.6 (5.3) | 26.3 (4.8)* | 32.1 (6.1) | 33.8 (6.0) | 35.8 (7.2) | 33.6 (6.3)* |
| HbA1c, mmol/mol† | 64 (13) | 64 (14) | 64 (14) | 64 (14) | 68 (15) | 68 (15) | 67 (12) | 68 (15) |
| HbA1c, % | 8.0 (1.2) | 8.0 (1.3) | 8.0 (1.3) | 8.0 (1.3) | 8.4 (1.4) | 8.4 (1.4) | 8.3 (1.1) | 8.4 (1.4) |
| Fasting plasma glucose, mmol/l† | 8.9 (3.3) | 9.3 (3.7) | 7.8 (3.0) | 9.1 (3.8) | 9.5 (2.6) | 9.7 (2.9) | 11.9 (4.4) | 9.9 (3.0) |
| Fasting plasma glucose, mg/dl | 161.3 (59.6) | 167.6 (67.0) | 140.4 (54.7) | 163.4 (68.6) | 172.0 (47.5) | 174.2 (51.6) | 214.5 (79.7) | 178.9 (54.4) |
| Type of insulin before insulin degludec initiation, n (%) | ||||||||
| Basal only | 1 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 67 (25.9) | 94 (22.7) | 22 (23.2) | 187 (22.4) |
| Basal + prandial | 887 (99.9) | 726 (100.0) | 53 (100.0) | 1693 (98.6) | 192 (74.1) | 321 (77.3) | 73 (76.8) | 621 (74.5) |
| Frequency of basal injections, n (%) | ||||||||
| Once daily | 675 (76.0) | 150 (20.7) | 19 (35.8) | 857 (49.9) | 201 (77.6) | 242 (58.3) | 53 (55.8) | 520 (62.4) |
| Twice daily or more | 202 (22.7) | 538 (74.1) | 34 (64.2) | 786 (45.8) | 54 (20.8) | 128 (30.8) | 40 (42.1) | 234 (28.1) |
| Unknown/missing | 11 (1.2) | 38 (5.2) | 0 (0.0) | 74 (4.3) | 4 (1.5) | 45 (10.8) | 2 (2.1) | 79 (9.5) |
| History of diabetes | ||||||||
| Duration of diabetes, years | 21.1 (13.5) | 22.9 (13.3) | 17.5 (11.8) | 21.8 (13.5) | 16.7 (8.2) | 17.9 (8.4) | 18.0 (7.1) | 17.5 (8.1) |
| Duration of insulin treatment, years | 20.6 (13.6) | 22.3 (13.2) | 16.9 (11.9) | 21.2 (13.5) | 8.5 (5.9) | 9.9 (6.3) | 11.5 (6.8) | 9.7 (6.3) |
| Presence of ≥1 ‘at risk for severe hypoglycaemia’ criterion, n (%) | ||||||||
| Experienced ≥1 severe hypoglycaemic event within the last year | 105 (11.8) | 68 (9.4) | 5 (9.4) | 181 (10.5)* | 15 (5.8) | 5 (1.2) | – | 21 (2.5)* |
| Moderate chronic renal failure | 39 (4.4) | 57 (7.9) | 1 (1.9) | 99 (5.8)* | 38 (14.7) | 52 (12.5) | 19 (20.0) | 117 (14.0)* |
| Hypoglycaemic symptom unawareness (history of impaired automatic responses) as judged by the physician | 174 (19.6) | 135 (18.6) | 14 (26.4) | 325 (18.9)* | 34 (13.1) | 22 (5.3) | 5 (5.3) | 61 (7.3)* |
| For Type 1 diabetes: duration of diabetes >15 years | 542 (61.0) | 477 (65.7) | 26 (49.1) | 1071 (62.4)* | – | – | – | – |
| For Type 2 diabetes: duration of diabetes >5 years | – | – | – | – | 165 (63.7) | 301 (72.5) | 79 (83.2) | 599 (71.9)* |
Glargine U100, insulin glargine 100 units/mL; IDet, insulin detemir; NPH, neutral protamine Hagedorn.
Values are mean (sd) unless otherwise stated. *Not published previously. †Calculated, not measured.
Comparisons of investigated outcomes 6 and 12 months post‐switch vs pre‐switch
Glycaemic control
In the Type 1 diabetes group, participants switching to degludec from glargine U100 or IDet benefitted from a significant reduction in HbA1c at both 6 and 12 months (Fig. 1a); the HbA1c reduction in people switching to degludec from NPH was not significant at either 6 or 12 months (Fig. 1a). In the Type 2 diabetes group, there was a significant reduction in HbA1c at both 6 and 12 months, irrespective of previous basal insulins (Fig. 1b).
Figure 1.
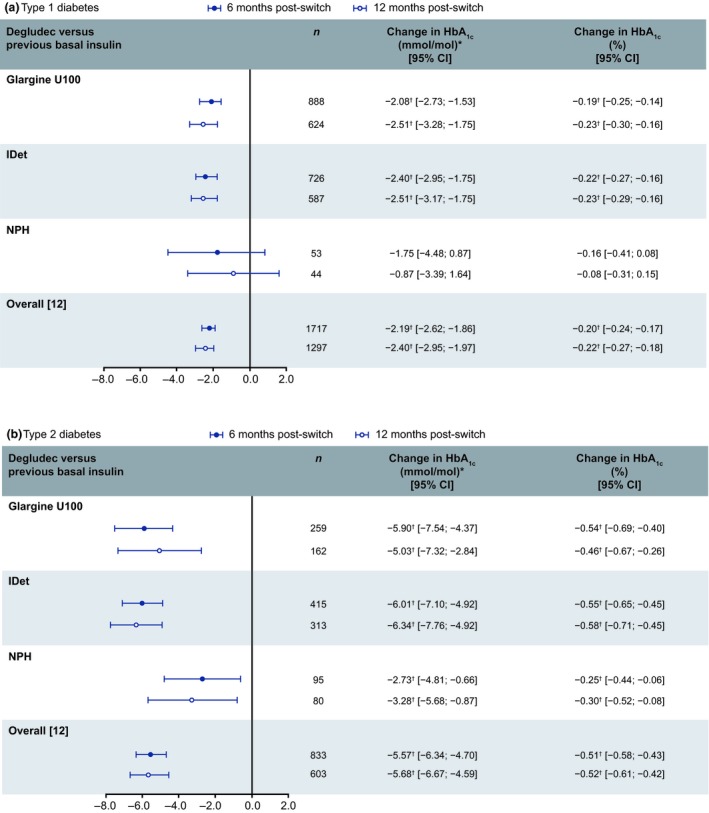
Change in HbA1c (%) in people with (a) Type 1 or (b) Type 2 diabetes who switched to degludec from other basal insulins at 6 and 12 months: post‐switch vs pre‐switch. *Calculated, not measured. † P<0.05. Data for the overall population were published in the primary manuscript 12; all changes were from baseline. Glargine U100, insulin glargine 100 units/mL; IDet, insulin detemir; n, number of participants; NPH, neutral protamine Hagedorn.
Hypoglycaemia
In Type 1 diabetes, switching to degludec from glargine U100 resulted in significantly lower rates of hypoglycaemia across all categories, at both 6 and 12 months post‐switch vs pre‐switch (Fig. 2a). Switching to degludec from IDet resulted in numerically, but not statistically significantly, lower rates of overall and overall non‐severe hypoglycaemia, and significantly lower rates of severe and nocturnal non‐severe hypoglycaemia, at both 6 and 12 months post‐switch vs pre‐switch (Fig. 2a). Switching to degludec from NPH resulted in numerically, but not statistically significantly, lower rates of overall and overall non‐severe hypoglycaemia at both 6 and 12 months, and a significantly lower rate of nocturnal non‐severe hypoglycaemia at 12 months post‐switch vs pre‐switch (Fig. 2a).
Figure 2.
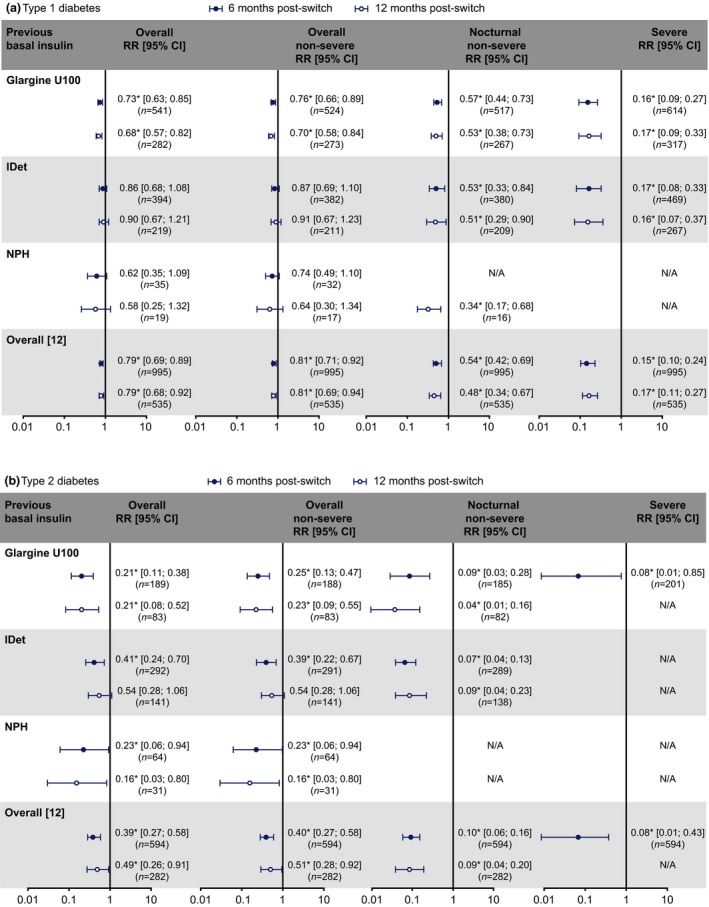
Rate ratios (RRs) of overall, overall non‐severe, nocturnal non‐severe and severe hypoglycaemia in people with (a) Type 1 or (b) Type 2 diabetes who switched to degludec from other basal insulins at 6 and 12 months: post‐switch vs pre‐switch. *P<0.05. Data for the overall population were published in the primary manuscript 12; all comparisons were based on similar 6‐ or 12‐month time frames, i.e. 0 to +6 vs −6 to 0 months, or 0 to +12 vs −12 to 0 months post‐ and pre‐switch, respectively. N/A, RRs could not be calculated because there were too few events in the post‐switch period. Glargine U100, insulin glargine 100 units/mL; IDet, insulin detemir; n, number of participants; NPH, neutral protamine Hagedorn.
In Type 2 diabetes, switching to degludec from glargine U100 resulted in significantly lower rates of hypoglycaemia across all categories at 6 months post‐switch vs pre‐switch (Fig. 2b); similar results were observed at 12 months, where comparisons were possible (Fig. 2b). People with Type 2 diabetes switching to degludec from IDet benefitted from significantly lower rates of overall, overall non‐severe and nocturnal non‐severe hypoglycaemia at 6 months (Fig. 2b); for the 12‐month comparisons, only the reduction in rate of nocturnal non‐severe hypoglycaemia was significant in the IDet group (Fig. 2b). In Type 2 diabetes, switching to degludec from NPH resulted in significantly lower rates of overall and overall non‐severe hypoglycaemia at both 6 and 12 months post‐switch vs pre‐switch (Fig. 2b).
For the comparisons where rate ratios could not be calculated because of the relatively low number of events in the post‐switch period (Figs 2a and b), the numbers of corresponding hypoglycaemic events after switching to degludec were numerically lower than those prior to switch. In addition, there was no significant effect of previous basal insulin type (IDet or glargine U100) on the 6‐month comparison in overall hypoglycaemia in the Type 1 (P=0.7905) or Type 2 diabetes group (P=0.1785), based on the Wald test.
Insulin dose and number of prandial insulin injections
In the Type 1 diabetes group, the daily doses of total, basal and prandial insulins decreased significantly at both 6 and 12 months irrespective of previous basal insulins, except for prandial insulin in the NPH group at 6 months, where there was a decrease that did not reach statistical significance (Fig. 3a). In Type 2 diabetes, there were no significant differences in total, basal or prandial insulin doses at 6 or 12 months post‐switch vs baseline, irrespective of previous basal insulins, except that the decrease in prandial insulin after switching to degludec reached statistical significance at 12 months in the overall population (Fig. 3b).
Figure 3.
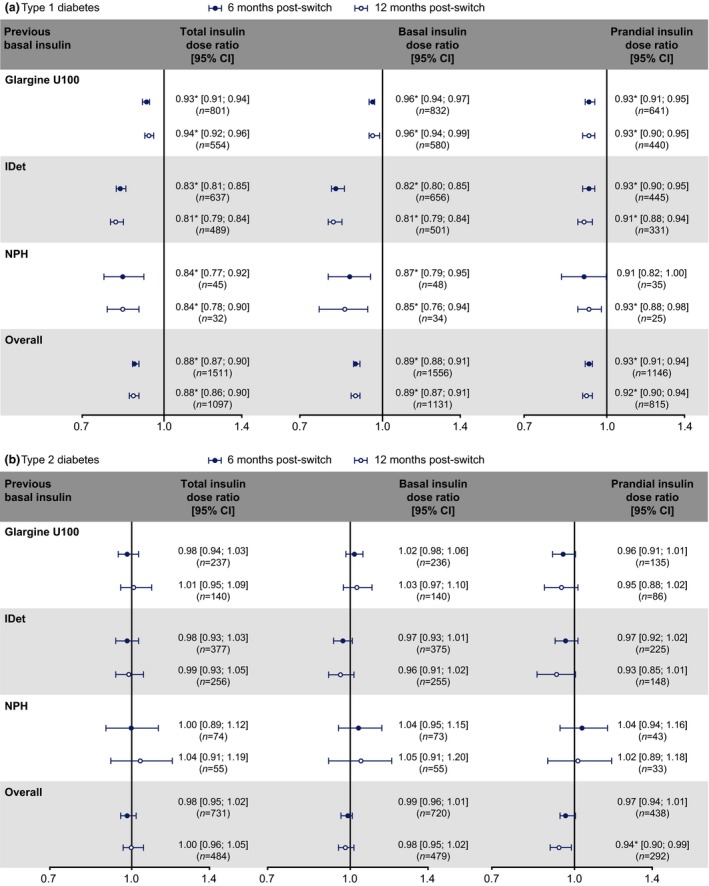
Dose ratios of total, basal and prandial insulins in people with (a) Type 1 or (b) Type 2 diabetes who switched to degludec from other basal insulins at 6 and 12 months post‐switch vs pre‐switch. *P<0.05. Dose ratios were based on 6 or 12 months post‐switch vs baseline. Glargine U100, insulin glargine 100 units/mL; IDet, insulin detemir; n, number of participants; NPH, neutral protamine Hagedorn.
In Type 1 diabetes, prior to switching to degludec, the mean numbers of daily prandial insulin injections were similar for all groups (glargine U100: 3.1; IDet: 3.2; NPH: 3.2; overall: 3.1). These values remained similar at 6 and 12 months post‐switch. In the Type 2 diabetes group, prior to switching to degludec, the mean number of daily injections for prandial insulin was 3.1 for all groups, and this remained similar at 6 and 12 months post‐switch.
Discussion
The present post hoc analysis of participants in EU‐TREAT demonstrated that switching basal insulin to degludec can lead to improvements in glycaemic control and a reduced risk of hypoglycaemia with a lower (Type 1 diabetes group) or similar (Type 2 diabetes group) insulin dose in routine clinical practice.
The results from the present study are consistent with those observed in the BEGIN trial programme, SWITCH 1 and 2, DEVOTE and small‐scale observational studies 7, 8, 9, 10, 11, 15. The nonsignificant HbA1c reduction in people with Type 1 diabetes switching to degludec from NPH at either 6 or 12 months was probably attributable to the relatively low number of people (n=53) in this group, as evidenced by the wide CIs (Fig. 1); however, the numerical HbA1c reduction in Type 1 diabetes and statistically significant HbA1c reduction in Type 2 diabetes at both 6 and 12 months indicate that switching to degludec from NPH generally improves glycaemic control. In terms of insulin treatment, basal insulin analogues are widely preferred over NPH because of their pharmacokinetic and pharmacodynamic advantages, including longer duration of action, less pronounced peak in time–action profiles and lower within‐subject variability, as well as decreased risk of hypoglycaemia 16. The results of the present study show the clinical benefits of switching to degludec not only from NPH, but also from glargine U100 and IDet.
The reduced risk of hypoglycaemia after switching to degludec from previous basal insulins, despite the lower HbA1c, is possibly explained by the flatter time–action profile of degludec that helps to reduce the magnitude and frequency of blood glucose fluctuations relative to other insulins 12. It is speculated that people on degludec subsequently spend more time with blood glucose within the target range, avoiding the peaks and nadirs associated with hyperglycaemia and hypoglycaemia, respectively 12. The results of the present study also indicate that in Type 1 diabetes, a lower dose of degludec was required to achieve improved glycaemic control in a real‐world population. In light of similar observations of a lower total, basal and prandial insulin dose in a basal–prandial trial comparing degludec with glargine U100 17, the need and opportunity to optimize both basal and prandial insulins individually should be considered, as there may be scope for lowering the risk of insulin‐induced hypoglycaemia.
For people switching from NPH insulin, several rate ratios for hypoglycaemia could not be calculated because there were too few events in the post‐switch period in this relatively small cohort. Switching to degludec from NPH, however, resulted in numerically reduced rates in Type 1 diabetes and significantly reduced rates in Type 2 diabetes, with regard to overall and overall non‐severe hypoglycaemia at both 6 and 12 months.
Hypoglycaemia is associated with various complications, including cognitive dysfunction, coma, seizures, falls and fractures, increased risk of cardiovascular events and mortality, significant negative impacts on health and quality of life 18, 19, 20, and a substantial cost burden to healthcare systems 21, 22, 23. Compounding this, the fear of hypoglycaemia can reduce people's adherence to insulin regimens 24 and lead to physicians setting less ambitious glycaemic targets 25. The lowered risk of hypoglycaemia and HbA1c observed in the present study, achieved with a lower insulin dose in Type 1 diabetes and similar dose in Type 2 diabetes after switching to degludec, indicates that degludec has the potential to reduce these burdens where the need exists, irrespective of previous basal insulin.
Limitations of the present analysis include those of the study previously reported, such as its observational, retrospective design and the absence of a comparator arm 12. In addition, participants in EU‐TREAT were empirically chosen, as people were switched to degludec because of difficulties while on their previous regimens, and demographic differences such as duration of diabetes and insulin treatment could potentially influence the risk of hypoglycaemia and response to the treatment change. A longer‐term study of degludec in clinical practice is needed to confirm that the observed benefits with degludec in EU‐TREAT are maintained beyond 12 months. A larger population in each previous basal insulin group may be beneficial in order to provide sufficient data to calculate the missing rate ratios of hypoglycaemia in the present analysis.
The strengths of EU‐TREAT have been reported previously, including the large‐scale, multicentre/country design, the use of real‐world data, and all treatment decisions being made independently of the study 12. Additional strengths of the present study include the consistency in direction and magnitude of the results at 6 and 12 months, and in two different diseases (Type 1 and Type 2 diabetes) and three previous basal insulin groups (glargine U100, IDet and NPH), which support the conclusion that the changes in clinical outcomes were robust observations.
In conclusion, the present study demonstrated the homogeneity of the clinical benefits obtained after switching to degludec from different basal insulins, suggesting that, irrespective of previous basal insulins, switching to degludec is associated with improved glycaemic control and lowered risk of hypoglycaemia in people with diabetes (non‐significant reduction in HbA1c and hypoglycaemia rates for the small NPH group in Type 1 diabetes), under routine clinical care. These benefits can be achieved with lower or similar total, basal and prandial insulin doses.
Funding sources
EU‐TREAT and this secondary analysis were funded by Novo Nordisk, which had a role in reviewing this manuscript for medical accuracy.
Competing interests
S.T.K. has served on the advisory panels for Novo Nordisk A/S, Merck, Sanofi‐Aventis, Eli Lilly, AstraZeneca and Boehringer Ingelheim, and the speakers' bureaus for Merck, AstraZeneca and Boehringer Ingelheim. A.L. has no financial disclosures to declare. B.S. has served on the advisory panels for Novo Nordisk A/S, Sanofi, AstraZeneca, Eli Lilly, Bayer and Boehringer Ingelheim, and speakers' bureaus for Sanofi, Eli Lilly, Merck Sharp Dohme (MSD), Bayer and Boehringer Ingelheim. N.T. has served on advisory panels for Merck Sharp Dohme, AstraZeneca, Sanofi, Novo Nordisk, ELPEN, Eli Lilly, Boehringer Ingelheim and Novartis, and has received research support from Merck Sharp Dohme, Eli Lilly, Novo Nordisk A/S, Sanofi, Pfizer, AstraZeneca, Janssen, Cilag, GlaxoSmithKline and Novartis. A.C. is an employee of Novo Nordisk A/S and his spouse is an employee of Novo Nordisk Region Europe Pharmaceuticals A/S. M.L.W. is an employee of and stockholder in Novo Nordisk A/S. T.S. has served on advisory panels for Abbott, Ascensia, Bayer Vital, Boehringer Ingelheim, Eli Lilly, Janssen, Medtronic, Merck Sharp Dohme, Novo Nordisk A/S and Sanofi, has received research support from AstraZeneca/Bristol‐Myers Squibb, Becton Dickinson, Eli Lilly, Merck Sharp Dohme, Novartis, Novo Nordisk A/S and Sanofi, and has served on speakers' bureau for Abbott, AstraZeneca/Bristol‐Myers Squibb, Berlin Chemie, Boehringer Ingelheim, Eli Lilly, Medtronic, Merck Sharp Dohme, Novartis, Novo Nordisk A/S and Sanofi.
Acknowledgements
The authors would like to thank Steffen Haldrup (Novo Nordisk at the time of the study) and Deniz Tutkunkardas (Novo Nordisk) for their review of and input to the manuscript. Medical writing assistance and editorial support were provided by Jin Heppell and Izabel James, of Watermeadow Medical, an Ashfield Company, part of UDG Healthcare plc. This support was funded by Novo Nordisk.
All authors had full access to the data and shared final responsibility for the content of the manuscript and decision to submit for publication. The participant‐level analysis datasets for the research presented in the publication are available from the corresponding author on reasonable request.
Diabet. Med. 36: 868–877 (2019)
(Clinical trials registry no.: NCT02662114)
References
- 1. The Diabetes Control and Complications Trial Research Group . Hypoglycemia in the Diabetes Control and Complications Trial.. Diabetes 1997; 48: 271–286. [PubMed] [Google Scholar]
- 2. Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes 2008; 57: 3169–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khunti K, Alsifri S, Aronson R, Cigrovski Berkovic M, Enters‐Weijnen C, Forsen T et al Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin‐treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab 2016; 18: 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady‐state conditions in type 1 diabetes. Diabetes Obes Metab 2012; 14: 859–864. [DOI] [PubMed] [Google Scholar]
- 5. Heise T, Nørskov M, Nosek L, Kaplan K, Famulla S, Haahr HL. Insulin degludec: Lower day‐to‐day and within‐day variability in pharmacodynamic response compared with insulin glargine 300 U/mL in type 1 diabetes. Diabetes Obes Metab 2017; 19: 1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tresiba® Summary of product characteristics. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002498/WC500138940.pdf. Last accessed 23 March 2018.
- 7. Ratner RE, Gough SCL, Mathieu C, Del Prato S, Bode B, Mersebach H et al Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre‐planned meta‐analysis of phase 3 trials. Diabetes Obes Metab 2013; 15: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies M, Sasaki T, Gross JL, Bantwal G, Ono Y, Nishida T et al Comparison of insulin degludec with insulin detemir in type 1 diabetes: a 1‐year treat‐to‐target trial. Diabetes Obes Metab 2016; 18: 96–99. [DOI] [PubMed] [Google Scholar]
- 9. Lane W, Bailey TS, Gerety G, Gumprecht J, Philis‐Tsimikas A, Hansen CT et al Effect of Insulin Degludec vs Insulin Glargine U100 on Hypoglycemia in Patients With Type 1 Diabetes: The SWITCH 1 Randomized Clinical Trial. JAMA 2017; 318: 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wysham C, Bhargava A, Chaykin L, de la Rosa R, Handelsman Y, Troelsen LN et al Effect of Insulin Degludec vs Insulin Glargine U100 on Hypoglycemia in Patients With Type 2 Diabetes: The SWITCH 2 Randomized Clinical Trial. JAMA 2017; 318: 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, Pieber TR et al Efficacy and Safety of Degludec versus Glargine in Type 2 Diabetes. N Engl J Med 2017; 377: 723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siegmund T, Tentolouris N, Knudsen ST, Lapolla A, Prager R, Phan TM et al A European, multicentre, retrospective, non‐interventional study (EU‐TREAT) of the effectiveness of insulin degludec after switching basal insulin in a population with type 1 or type 2 diabetes. Diabetes Obes Metab 2018; 20: 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Medical Association . Declaration of Helsinki‐Ethical Principles for Medical Research Involving Human Subjects. Last amended by the 64th WMA Gemeral Assembly, Fortaleza, Brazel, October 2013. Available at https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Last accessed 11 September 2018.
- 14. REPEATED Statement, The GENMOD Procedure, SAS/STAT(R) 9.3 User's Guide. Available at https://support.sas.com/documentation/cdl/en/statug/63962/HTML/default/viewer.htm#statug_genmod_sect029.htm. Last accessed January 2019.
- 15. Landstedt‐Hallin L. Changes in HbA1c, insulin dose and incidence of hypoglycemia in patients with type 1 diabetes after switching to insulin degludec in an outpatient setting: an observational study. Curr Med Res Opin 2015; 31: 1487–1493. [DOI] [PubMed] [Google Scholar]
- 16. Poon K, King AB. Glargine and detemir: Safety and efficacy profiles of the long‐acting basal insulin analogs. Drug Healthc Patient Saf 2010; 2: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heller S, Buse J, Fisher M, Garg S, Marre M, Merker L et al Insulin degludec, an ultra‐longacting basal insulin, versus insulin glargine in basal‐bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal‐Bolus Type 1): a phase 3, randomised, open‐label, treat‐to‐target non‐inferiority trial. Lancet 2012; 379: 1489–1497. [DOI] [PubMed] [Google Scholar]
- 18. Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol 2014; 10: 711. [DOI] [PubMed] [Google Scholar]
- 19. Frier BM, Schernthaner G, Heller SR. Hypoglycemia and Cardiovascular Risks. Diabetes Care 2011; 34: S132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, Diabetes, and Cardiovascular Events. Diabetes Care 2010; 33: 1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. The DCCT Research Group. Am J Med 1991; 90: 450–459. [PubMed] [Google Scholar]
- 22. Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011; 365: 2002–2012. [DOI] [PubMed] [Google Scholar]
- 23. Harris SB, Leiter LA, Yale JF, Chiasson J‐L, Kleinstiver P, Sauriol L. Out‐of‐pocket costs of managing hyperglycemia and hypoglycemia in patients with type 1 diabetes and insulin‐treated type 2 diabetes. Can J Diabetes 2007; 31: 25–33. [Google Scholar]
- 24. Fidler C, Elmelund Christensen T, Gillard S. Hypoglycemia: An overview of fear of hypoglycemia, quality‐of‐life, and impact on costs. J Med Econ 2011; 14: 646–655. [DOI] [PubMed] [Google Scholar]
- 25. Peyrot M, Barnett AH, Meneghini LF, Schumm‐Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med 2012; 29: 682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]


