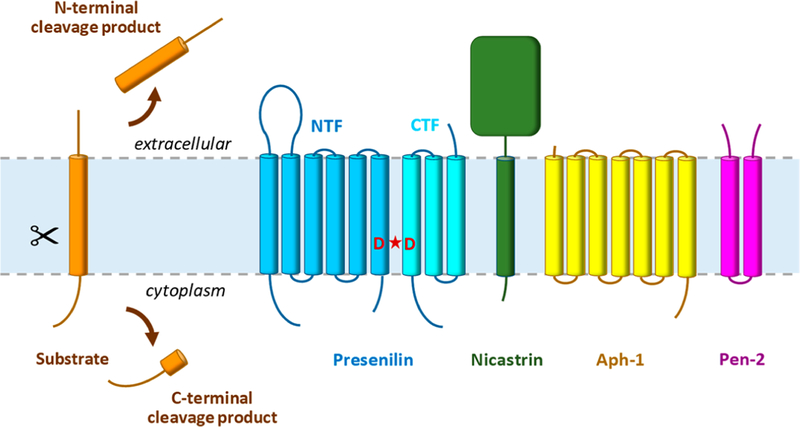
Figure 1.
Intramembrane proteolysis by the γ-secretase complex. γ-Secretase cleaves >90 different type I integral membrane proteins after ectodomain release by membrane-tethered sheddases. The protease complex carries out proteolysis in the transmembrane domain of these substrates to secrete N-terminal cleavage products into the extracellular milieu and release C-terminal cleavage products into the cytoplasm. The γ-secretase complex is composed of four different multipass membrane proteins, with presenilin as the catalytic component containing two transmembrane aspartates in the active site. Upon assembly with the other subunits (nicastrin, Aph-1, and Pen-2), presenilin undergoes autoproteolysis into an N-terminal fragment (NTF) and C-terminal fragment (CTF) to form active γ-secretase.