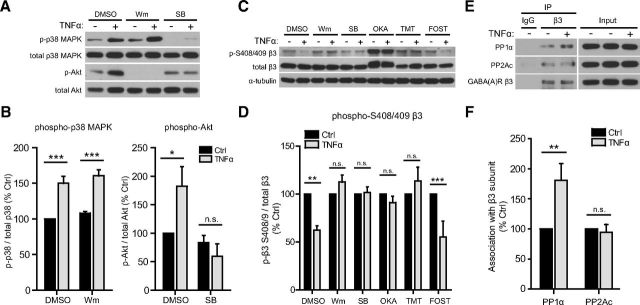
Figure 7.
TNFα dephosphorylates GABAAR β3 S408/409 by sequentially activating p38 MAPK and PI3K and enhancing the association of PP1α with β3 subunits. A, Representative Western blots of phospho- p38 MAPK, total p38 MAPK, phospho-Akt, and total Akt in lysates from control or TNFα-treated (100 ng/ml, 45 min) cultures, preincubated with DMSO (solvent, 0.1%) or inhibitors of PI3K [wortmannin (Wm), 100 nm] and p38 MAPK [SB 202190 (SB), 10 μm]. B, Quantification data showing an increase in phospho-p38 MAPK with TNFα treatment, unaffected by PI3K inhibition (left), and an increase in phosphorylation of the PI3K downstream effector Akt, blocked by p38 MAPK inhibition (right) (n = 4–5 independent experiments; Mann–Whitney U test, *p < 0.05, **p < 0.01; n.s., not significant). C, Representative Western blots of phospho-S408/409 GABAAR β3, total GABAAR β3, and α-tubulin in lysates from control or TNFα-treated (100 ng/ml, 45 min) cultures, preincubated with DMSO (solvent, 0.1%) or inhibitors of PI3K [wortmannin (Wm), 100 nm], p38 MAPK [SB 202190 (SB), 10 μm], PP1/PP2A [okadaic acid (OKA), 0.5 μm], PP1 [tautomycetin (TMT), 10 nm], and PP2A [fostriecin (FOST), 10 nm]. D, Quantification data showing a decrease in GABAAR β3 phospho-S408/409, blocked by inhibition of PI3K, p38 MAPK, and PP1, but not by inhibition of PP2A (n = 5 independent experiments; Mann–Whitney U test, **p < 0.01, ***p < 0.001; n.s., not significant). E, Representative Western blots of coimmunoprecipitation experiments in which the GABAAR β3 subunit was immunoprecipitated (IP) from untreated (Ctrl) or TNFα-treated (100 ng/ml, 30 min) cultures; blots were then probed for GABAAR β3 and for the catalytic subunits of PP1 (PP1α) and PP2A (PP2Ac). F, Quantification showing that TNFα treatment enhances the association of PP1α but not PP2Ac with β3 subunits (n = 5–6 independent experiments; Mann–Whitney U test, **p < 0.01; n.s., not significant).