Figure 5.
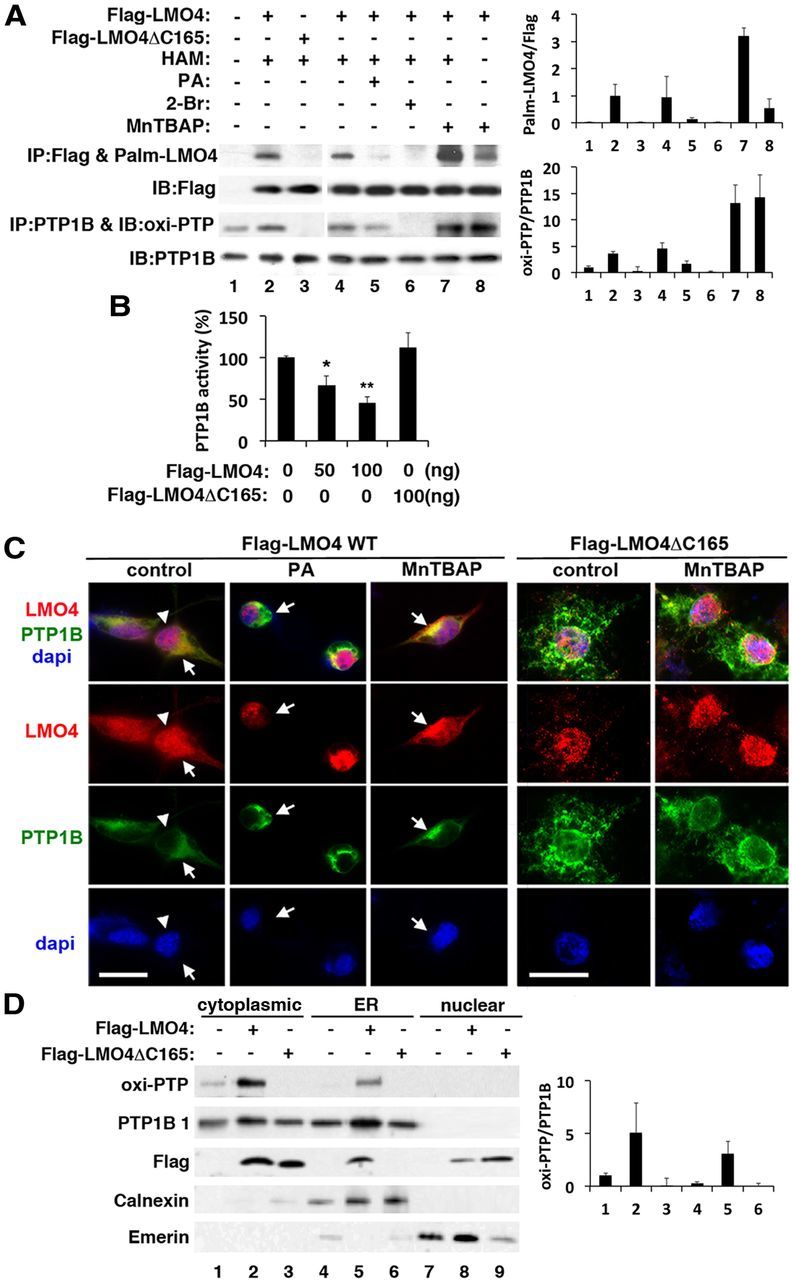
Palmitoylation of LMO4 regulates its subcellular localization and inhibition of PTP1B activity. A, Immunoblot (IB) analysis reveals that LMO4 is palmitoylated (Palm) and correlates with oxi-PTP1B levels. Lane 7 is a negative control that leaves out hydroxylamine (HAM) from the assay. Palmitic acid (PA; 400 μm), 2-bromo-hexadecanoic acid (2-Br; 50 μm), and MnTBAP (10 μm) were applied to cells for 6 h. B, PTP1B activity is suppressed by overexpression of WT but not mutant (ΔC165) LMO4. *p < 0.05 compared with baseline; **p < 0.05 compared with the previous value. n = 6 per group. C, Palmitoylation controls cytoplasmic retention of LMO4. YFP-tagged PTP1B was present in the cytoplasm at the ER. The palmitoylation-defective mutant LMO4ΔC165 was detected in the nucleus even in the presence of MnTBAP. Colocalization of LMO4 (red) with PTP1B (green) appears yellow. Nuclei are revealed by DAPI staining (blue). Scale bars, 15 μm. D, Immunoblot analysis confirmed the presence of Flag–LMO4 and PTP1B in the ER. Calnexin is an ER marker, and emerin is a nucleus-enriched marker. Flag–LMO4ΔC165 was not retained in the ER fraction but was present in the nuclear fraction. IP, Immunoprecipitation.
