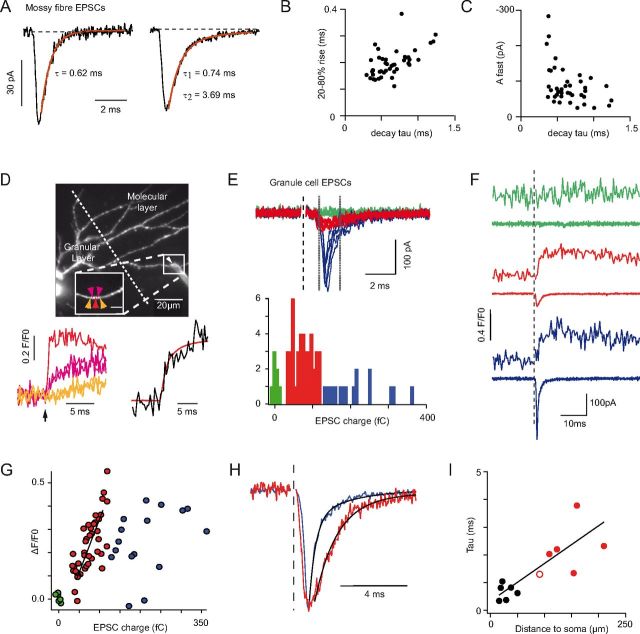
Figure 6.
The decay kinetics of mf-EPSCs and GrC-EPSCs, and the relationship between EPSC decay time and synapse distance from the GoC soma. A, Unitary mf-EPSCs evoked by minimal WM stimulation, from two different cells. Black traces represent individual trials. Red traces represent best fits with decaying exponentials. Left, Monoexponentially decaying EPSC (average of 50 trials). Right, Biexponentially decaying EPSC (Aslow /Afast = 0.19; average of 20 trials). Most (43 of 48) cells displayed biexponential mf-EPSC decay. B, 20–80% rise time of unitary mf-EPSCs versus the respective fast decay time constant, for 43 biexponentially decaying cells. Rise and decay are positively correlated (r = 0.55, p < 0.001, Spearman rank correlation). C, In the same cells, amplitude of the unitary mf-EPSC fast component (Afast) versus the respective time constant (τfast). Afast and τfast are negatively correlated (r = −0.41, p < 0.01). D, Projected morphology of a GoC obtained by multiphoton imaging of Alexa-594 introduced through the recording pipette in a parasagittal slice. Apical dendrites ascending to the molecular layer cross the Purkinje cell layer (dotted line indicates the apex of Purkinje cells). A stimulation electrode (white arrowhead) was placed at the ML surface above a deep-running dendrite (boxed region, inset). Ca2+ influx was imaged quasi-simultaneously at POIs indicated by colored arrows in the inset. Lower left panel, The corresponding fluorescence traces are displayed (color coded; average of 65 stimulations). Black arrow under the traces represent stimulation time. Lower right, Sum of the fluorescence signal over all responding points (black trace), representing the total Ca2+ influx. The charge integral of the average AMPA EPSC (red trace) is superimposed on the fluorescence Ca2+ transient. There is similarity in their rise kinetics. E, Top, Electrophysiological traces with evoked EPSCs. Bottom, Histogram of EPSC charges (measured between the two vertical lines in the top traces). Three types of responses can be discriminated based on the magnitude of the charge integral: failures (0–15 fC; green traces; n = 7), low amplitude slow events (15–120 fC; red traces; n = 42), and large amplitude fast events (>120 fC; blue traces; n = 16). F, Average Ca2+ transients (top traces; averaged for 5 POIs around the entry site) and corresponding EPSCs (bottom traces) for the three types of responses in E. G, Correlation between the EPSC charge (measured between the dotted lines of E) and the Ca2+ influx (ΔF/F0) recorded in the apical dendrite underlying the stimulation electrode. ΔF/F0 here is the average over 1.45 ms around the peak for the sum of all responding POIs. The charge of small EPSCs is significantly correlated to Ca2+ influx (red points and black line; Pearson's correlation coefficient 0.793; p < 0.001; n = 42), indicating a synaptic contact at the imaging site. Large EPSCs are not correlated with Ca2+ influx (blue points; Pearson's correlation coefficient 0.371; p > 0.1; n = 16), indicating that large EPSCs do not occur at the Ca2+ imaging site but may crown smaller EPSCs occurring at this site, which are responsible for the Ca2+ influx. H, Peak-scaled averaged EPSCs showing that the local pf events (red) decay with slower kinetics than the distal aa events (blue). Black lines indicate biexponential fits to the decay phase (blue EPSC, first component: 0.6 ms; red EPSC, first component: 1.3 ms). I, First decay time constant of pf-EPSCs (n = 6; red symbols) and mf-EPSCs (n = 6; black symbols) as a function of the distance of the synaptic site (identified by Ca2+ imaging) from the soma. The hollow red circle represents the experiment illustrated in this figure (D–H).