Figure 3.
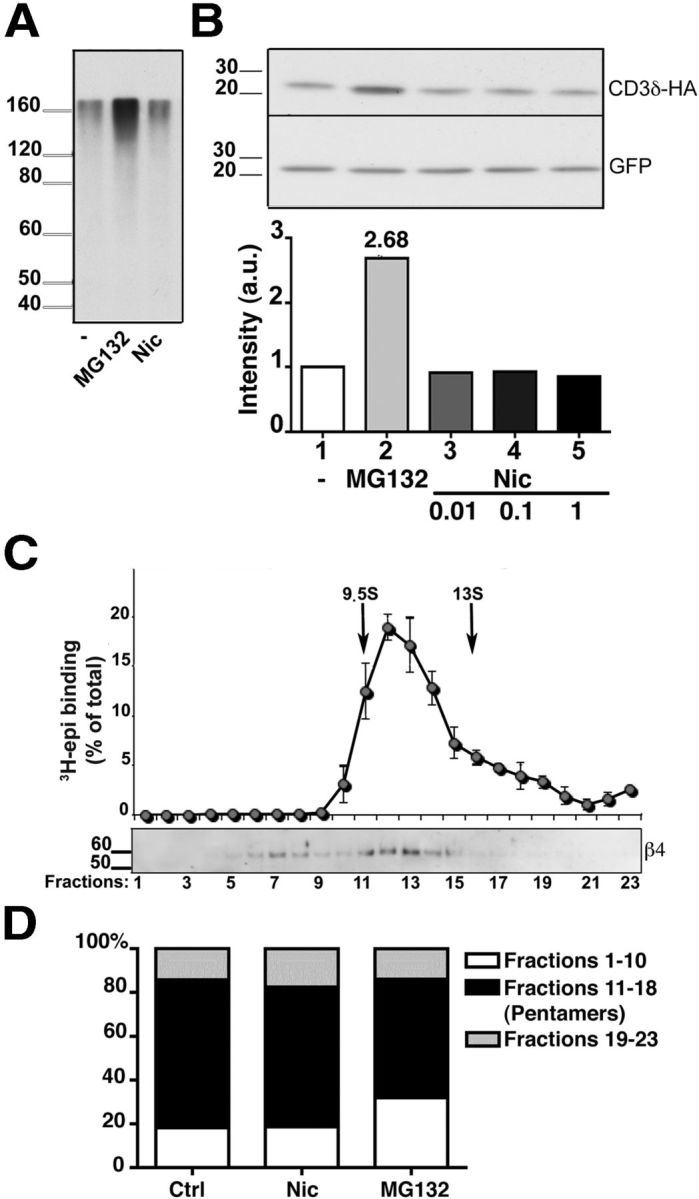
Effect of nicotine on proteasomal activity and on α3β4 receptor assembly. A, B, Nicotine does not impair proteasome activity. A, HeLa cells were incubated with 50 μg/ml of CHX for 3 h in the presence of 10 μm MG132, 1 mm nicotine, or no additional drug, as indicated. The presence of polyubiquitinated proteins was then analyzed by Western blotting with anti-ubiquitin antibodies. B, The degradation of the ERAD substrate CD3δ is not affected by the presence of nicotine. HeLa cells were transfected with HA-tagged CD3δ and GFP cDNAs. After 24 h, cells were incubated for 3 h with 50 μg/ml of CHX either alone, or together with 10 μm MG132, or with the indicated concentration of nicotine. Cell lysates were then analyzed by Western blotting, using anti-HA and anti-GFP antibodies. C, D, α3β4 receptors are assembled efficiently into pentamers. C, Sedimentation analysis of α3β4 receptors from transfected cells treated for 8 h with MG132. Detergent extracts were analyzed as in Figure 1. D, Extent of pentamer assembly in untreated, nicotine-treated (1 mm; 24 h), or MG132-treated (10 μm; 8 h) cells. The histograms represent the percentage of material sedimenting more slowly than pentamers (fractions 1–10), as pentamers (fractions 11–18), and as aggregates (fractions 19–23) detected by Western blot with anti β4 antibodies (n = 4, 3, and 5 for control, nicotine-, and MG132-treated cells, respectively).
