Figure 1.
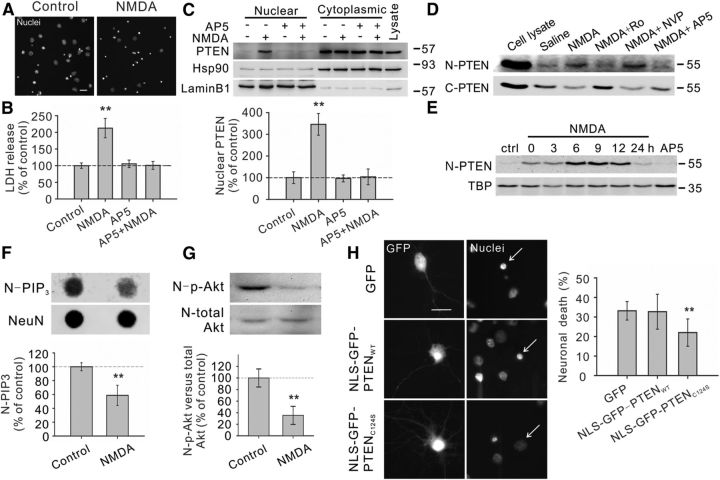
Excitotoxic NMDA stimulation increases PTEN nuclear translocation in cultured neurons. A, B, Bath application of NMDA (25 μm for 1 h, plus 6 h recovery) produced significant neuronal death, as evidenced by the increased numbers of neurons displaying condensed/fragmented nuclei. Scale bar, 20 μm. (Fig. 2C, for quantification) and the elevated extracellular levels of LDH (B, n = 7 culture dishes per group, from 7 independent experiments). The enhanced LDH release could be completely blocked by coapplication of the NMDAR antagonist AP5 (50 μm). C, Western blotting of the cytoplasmic and nuclear fractions showed that NMDA stimulation (25 μm for 1 h, plus 6 h recovery) enhanced PTEN translocation into the nucleus, a process that could be inhibited by AP5 (50 μm). We confirmed the quality of the cytoplasm/nuclei fractionation by sequentially reprobing the blots with cytoplasmic marker Hsp90 and nuclear marker lamin B1. Bar graph at the bottom summarizes data from four independent experiments. D, Western blotting of the nuclear (N-PTEN) and cytoplasmic (C-PTEN) fractions demonstrated that this enhanced PTEN nuclear translocation is primarily mediated by NR2B-containing NMDARs (NR2BRs), as this process could be blocked by NR2B antagonist Ro25-6981 (Ro; 0.5 μm), but not with NR2A-containing NMDAR preferring antagonist NVP-AAM077 (NVP; 0.4 μm). E, Sequential probing of nuclear fractions for PTEN (N-PTEN) and nuclear marker (TBP) showed that the NMDA-induced PTEN nuclear translocation was time-dependent, reaching a peak 6–9 h after stimulation and returning to baseline levels within 24 h; n = 2 culture dishes per group, from two independent experiments. It is worth noting that the time course of NMDA-induced PTEN nuclear translocation is slightly different from that induced by ischemia (Fig. 3A), probably due to different stimulation intensity. F, Sequential dot blotting of nuclear fractions for PIP3 (N-PIP3) and nuclear protein NeuN (NeuN) revealed that the NMDA stimulation (25 μm for 1 h, plus 6 h recovery) caused a marked drop in the nuclear levels of PIP3, the major physiological substrate of PTEN; n = 5 culture dishes per group. G, Sequential blotting of nuclear fractions for phospho-AktSer473 (N-p-Akt) and total Akt (N-total Akt) revealed that the NMDA treatment (25 μm for 1 h, plus 9 h recovery) caused a dramatic reduction in the nuclear levels of phospho-AktSer473; n = 3 culture dishes per group. H, Nuclear PTEN activity is critical for NMDA-induced neuronal damage. Images (left) and quantification of neuronal death (right) revealed that NMDA-induced neuronal death, assayed by nuclear condensation at 6 h after NMDA stimulation (25 μm for 1 h), was significantly reduced by overexpression of nucleus-targeted phosphatase-dead PTEN mutant (NLS-GFP-PTENC124S), but not by overexpression of either GFP or nucleus-targeted wild-type PTEN (NLS-GFP-PTENWT); n = 12 culture wells in each group, 25 neurons in each culture well were counted, from three independent experiments. Scale bar, 20 μm. Bars represent the group means, and error bars represent SD. Post hoc analysis compares treatment groups with control group after significant one-way ANOVA or Kruskal–Wallis on ranks, and significance is defined as *p < 0.05, **p < 0.01.