Figure 3.
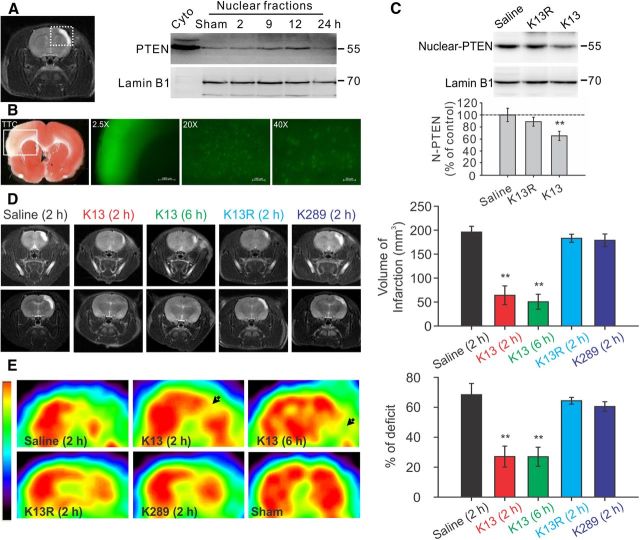
Enhanced PTEN nuclear translocation contributes to ischemic neuronal injuries following a focal ischemic brain insult in vivo. Mature Sprague Dawley rats were challenged with a 90 min focal ischemic event by a transient ligation of the right MCA and bilateral CCAs, and then allowed to recover for various periods of time as indicated. A, Ischemic insult triggered a time-dependent PTEN nuclear translocation in the infarct cortical area. Left, Representative coronal scanning image of MRI taken at day 7 from a saline-treated control stroke rat showed a well defined infarct (white) area. Right, Sequential probing of nuclei fractions for PTEN and nuclear protein lamin B1 from the infarct areas (the white square area indicated on the left) taken at various time points following stroke, showed a time-dependent increase in PTEN nuclear translocation, which reached a peak 12 h after stroke and returned to baseline levels within 24 h (n = 2 rats per group). B, Systemic application of Tat-K13 effectively entered neurons in the brain, including neurons in the ischemic penumbra region. Fluorescent peptide Tat-K13-Carboryfluorescein (10 mg/kg, i.v.) was applied 6 h after stroke insult, and brain sections were processed 1 h after the peptide administration. Left, The image of triphenyltetrazolium chloride (TTC) staining (1-mm-thick coronal brain section) revealed an observable infarct area in the sensorimotor cortex in the insult side of the brain. Right, Fluorescent microscopic images were taken from sections immediately adjacent to the TTC stained section on the left. The image (taken at 2.5× magnification) of the infarct area in a 1-mm-thick brain section corresponds to the area indicated by the white box in the TTC section on the Left, and showed that the peptide not only entered the brain, but also appeared to be particularly enriched in the infarct region. Images of a 20-μm-thick brain section of the infarct region taken at high magnifications (20× or 40×) further confirmed the successful transduction of the peptide into individual cells in the ischemic area. C, Application of Tat-K13 peptide at 6 h after ictus reduced the stroke-triggered PTEN nuclear translocation. Sequential probing of nuclei fractions for PTEN (N-PTEN) and nuclear protein lamin B1 from the infarct regions showed that Tat-K13 (K13; 10 mg/kg, i.v.), but not its control Tat-K13R (K13R; 10 mg/kg, i.v.), significantly decreased the ischemia-induced PTEN nuclear translocation when assayed at 12 h after stroke (n = 6 rats per group). D, Representative MRI scanning images from rostral (top) to caudal (bottom) levels were taken at day 7 from rats subjected to a 90 min transient focal ischemic insults and received various treatments as indicated. Bar graph on the right summarizes results from 10 rats in each group. Tat-K13 (K13; 10 mg/kg, i.v.) given at either 2 or 6 h after stroke-onset significantly reduced infarct areas in comparison with the control peptide Tat-K13R (K13R; 10 mg/kg, i.v.) or Tat-K289 (K289; 10 mg/kg, i.v.), or saline administered at 2 h after stroke onset. E, FDG-PET scanning taken at day 7 from the same groups of animals in D revealed a striking recovery in brain glucose metabolic activities in the infarct areas of animals receiving Tat-K13 in comparison with that in animals receiving the control peptide Tat-K13R or Tat-K289, or saline. The color scale (left) represents relative levels of glucose metabolism, from high (red) to low (blue). Bars represent the group means, and error bars represent SD. Post hoc analysis compares treatment groups with control (saline) group after significant one-way ANOVA or Kruskal–Wallis on ranks, and significance is defined as *p < 0.05, **p < 0.01.