Figure 1.
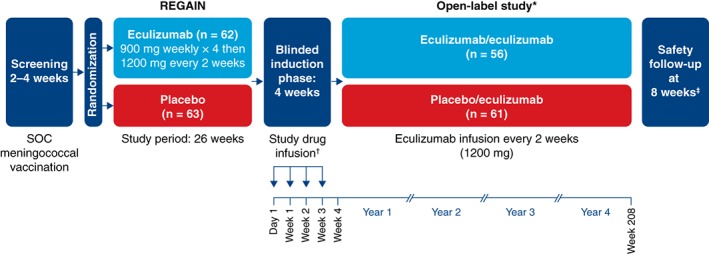
Study design. *Interim data are reported from the December 31, 2017 data cutoff point. †During the blinded induction phase of the open‐label study, patients received eculizumab (1,200 mg; 4 vials) at day 1 and week 2 and received placebo (4 vials) at weeks 1 and 3 (eculizumab/eculizumab group) or placebo (1 vial) plus eculizumab (900 mg; 3 vials) each week (placebo/eculizumab group). ‡Patients who withdrew or discontinued after receiving any amount of eculizumab were required to complete a safety follow‐up visit 8 weeks after their last eculizumab dose. SOC, standard of care.
